Thiazide Diuretic-Induced Change in Fasting Plasma Glucose: a Meta-analysis of Randomized Clinical Trials
- PMID: 32157653
- PMCID: PMC7280437
- DOI: 10.1007/s11606-020-05731-3
Thiazide Diuretic-Induced Change in Fasting Plasma Glucose: a Meta-analysis of Randomized Clinical Trials
Abstract
Background: Prior meta-analyses measuring thiazide-induced glycemic change have demonstrated an increased risk of incident diabetes; however, this measure's definition has changed over time.
Aim: To determine the magnitude of change in fasting plasma glucose (FPG) for thiazide diuretics.
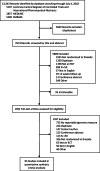
Data sources: A research librarian designed and conducted searches in Medline®, EMBASE, and EBM Reviews-Cochrane Central Register of Controlled Trials (inception through July 2018) and International Pharmaceutical Abstracts (inception to December 2014).
Study selection: Randomized, controlled trials comparing a thiazide or thiazide-like diuretic to any comparator reporting FPG were identified. Trials enrolling < 50 participants, those with a follow-up period of < 4 weeks, and conference abstracts were excluded.
Data extraction: Independent duplicate screening of citations and full-text articles, data extraction, and assessment of risk of bias was conducted.
Data synthesis: Ninety-five studies were included (N = 76,608 participants), with thiazides compared with placebo, beta-blockers, calcium channel blockers, renin-angiotensin-aldosterone-system inhibitors, potassium-sparing diuretic, and others alone or in combination. Thiazide diuretics marginally increased FPG (weighted mean difference 0.20 mmol/L (95% CI 0.15-0.25); I2 = 84%) (1 mmol/L = 18 mg/dL). Results did not change substantially when considering dose or duration, comparing thiazides with placebo or an active comparator, or using thiazides as monotherapy or combination therapy, even when combined with a potassium-correcting agent.
Conclusion: Thiazide diuretics have a small and clinically unimportant impact on FPG.
Keywords: fasting plasma glucose; meta-analysis; randomized controlled trial; thiazide diuretic.
Conflict of interest statement
No author has any financial arrangements or potential conflicts of interest to disclose with regard to products in this manuscript.
Figures


References
-
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can J Cardiol. 2018;34(5):506–525. - PubMed
-
- James PA, Oparil S, Carter BL, et al. Evidence-Based Guideline for the Management of High Blood Pressure in Adults. JAMA. 2014;311(5):507–520. - PubMed
-
- Carter BL, Einhorn PT, Brands M, et al. Thiazide-induced dysglycemia: Call for research from a working group from the national heart, lung, and blood institute. Hypertension. 2008;52(1):30–36. - PubMed
-
- Elliott WJ. Effects of potassium-sparing versus thiazide diuretics on glucose tolerance: New data on an old topic. Hypertension. 2012;59(5):911–912. - PubMed
-
- Eriksson JW, Jansson PA, Carlberg B, et al. Hydrochlorothiazide, but not candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: The Mechanisms for the Diabetes Preventing Effect of Candesartan (MEDICA) study. Hypertension. 2008;52(6):1030–1037. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

