β-Lapachone enhances the antifungal activity of fluconazole against a Pdr5p-mediated resistant Saccharomyces cerevisiae strain
- PMID: 32157667
- PMCID: PMC7455662
- DOI: 10.1007/s42770-020-00254-9
β-Lapachone enhances the antifungal activity of fluconazole against a Pdr5p-mediated resistant Saccharomyces cerevisiae strain
Abstract
Objectives: The aim of this study was to evaluate the ability of lapachones in disrupting the fungal multidrug resistance (MDR) phenotype, using a model of study which an azole-resistant Saccharomyces cerevisiae mutant strain that overexpresses the ATP-binding cassette (ABC) transporter Pdr5p.
Methods: The evaluation of the antifungal activity of lapachones and their possible synergism with fluconazole against the mutant S. cerevisiae strain was performed through broth microdilution and spot assays. Reactive oxygen species (ROS) and efflux pump activity were assessed by fluorometry. ATPase activity was evaluated by the Fiske and Subbarow method. The effect of β-lapachone on PDR5 mRNA expression was assessed by RT-PCR. The release of hemoglobin was measured to evaluate the hemolytic activity of β-lapachone.
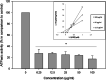
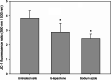
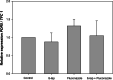
Results: α-nor-Lapachone and β-lapachone inhibited S. cerevisiae growth at 100 μg/ml. Only β-lapachone enhanced the antifungal activity of fluconazole, and this combined action was inhibited by ascorbic acid. β-Lapachone induced the production of ROS, inhibited Pdr5p-mediated efflux, and impaired Pdr5p ATPase activity. Also, β-lapachone neither affected the expression of PDR5 nor exerted hemolytic activity.
Conclusions: Data obtained indicate that β-lapachone is able to inhibit the S. cerevisiae efflux pump Pdr5p. Since this transporter is homologous to fungal ABC transporters, further studies employing clinical isolates that overexpress these proteins will be conducted to evaluate the effect of β-lapachone on pathogenic fungi.
Keywords: Fluconazole; Lapachone; Multidrug resistance; Yeast.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Histatin-5 induces the reversal of Pdr5p mediated fluconazole resistance in Saccharomyces cerevisae.J Mycol Med. 2018 Mar;28(1):137-142. doi: 10.1016/j.mycmed.2017.11.002. Epub 2017 Dec 6. J Mycol Med. 2018. PMID: 29217144
-
Synthetic organotelluride compounds induce the reversal of Pdr5p mediated fluconazole resistance in Saccharomyces cerevisiae.BMC Microbiol. 2014 Jul 26;14:201. doi: 10.1186/s12866-014-0201-y. BMC Microbiol. 2014. PMID: 25062749 Free PMC article.
-
Synergistic interactions between β-lapachone and fluconazole in the inhibition of CaCdr2p and CaMdr1p in Candida albicans.Rev Iberoam Micol. 2020 Jul-Oct;37(3-4):104-106. doi: 10.1016/j.riam.2020.09.002. Epub 2020 Nov 20. Rev Iberoam Micol. 2020. PMID: 33229297
-
Yeast ATP-binding cassette transporters conferring multidrug resistance.Annu Rev Microbiol. 2012;66:39-63. doi: 10.1146/annurev-micro-092611-150111. Epub 2012 Jun 11. Annu Rev Microbiol. 2012. PMID: 22703054 Review.
-
Characterization of the multi-drug efflux systems of pathogenic fungi using functional hyperexpression in Saccharomyces cerevisiae.Nihon Ishinkin Gakkai Zasshi. 2010;51(2):79-86. doi: 10.3314/jjmm.51.79. Nihon Ishinkin Gakkai Zasshi. 2010. PMID: 20467195 Review.
Cited by
-
Synthesis of Altissimacoumarin D and Other Prenylated Coumarins and Their Ability to Reverse the Multidrug Resistance Phenotype in Candida albicans.J Fungi (Basel). 2023 Jul 18;9(7):758. doi: 10.3390/jof9070758. J Fungi (Basel). 2023. PMID: 37504746 Free PMC article.
-
Digoxin Derivatives Sensitize a Saccharomyces cerevisiae Mutant Strain to Fluconazole by Inhibiting Pdr5p.J Fungi (Basel). 2022 Jul 25;8(8):769. doi: 10.3390/jof8080769. J Fungi (Basel). 2022. PMID: 35893137 Free PMC article.
-
Repurposing the FDA-approved anticancer agent ponatinib as a fluconazole potentiator by suppression of multidrug efflux and Pma1 expression in a broad spectrum of yeast species.Microb Biotechnol. 2022 Feb;15(2):482-498. doi: 10.1111/1751-7915.13814. Epub 2021 May 6. Microb Biotechnol. 2022. PMID: 33955652 Free PMC article.
-
Antifungal activity of β-lapachone against a fluconazole-resistant Candida auris strain.Braz J Microbiol. 2024 Sep;55(3):2593-2601. doi: 10.1007/s42770-024-01375-1. Epub 2024 May 14. Braz J Microbiol. 2024. PMID: 38743245 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

