Comparison of the Intraocular Pressure-Lowering Efficacy and Safety of the Brinzolamide/Brimonidine Fixed-Dose Combination versus Concomitant Use of Brinzolamide and Brimonidine for Management of Open-Angle Glaucoma or Ocular Hypertension
- PMID: 32158181
- PMCID: PMC6986681
- DOI: 10.2147/OPTH.S231402
Comparison of the Intraocular Pressure-Lowering Efficacy and Safety of the Brinzolamide/Brimonidine Fixed-Dose Combination versus Concomitant Use of Brinzolamide and Brimonidine for Management of Open-Angle Glaucoma or Ocular Hypertension
Abstract
Objective: To demonstrate that the intraocular pressure (IOP)-lowering efficacy of a twice-daily brinzolamide 10 mg/mL (BRINZ)/brimonidine 2 mg/mL (BRIM) fixed-dose combination (BBFC) was non-inferior to its individual components (BRINZ+BRIM) dosed concomitantly in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT). Safety was also evaluated.
Methods and analysis: This was a Phase III, multicenter, observer-masked study in patients from China, Russia and Taiwan. Patients aged ≥18 years with a mean IOP ≥21 mmHg and ≤36 mmHg in the same eye after washout of other IOP-lowering medications were included. Eligible patients were randomized (1:1) to receive BBFC or BRIZ+BRIM eye drops twice daily for 3 months. The primary endpoint was the mean change in diurnal IOP (averaged over 09:00, +2 h, and +7 h) from baseline to Month 3. Adverse events (AEs) were recorded throughout the study.
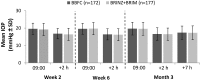
Results: The per-protocol set included 349 patients (BBFC, n=172; BRINZ+BRIM, n=177). The mean±standard deviation diurnal IOP at baseline was 24.6±2.66 mmHg in both groups. At Month 3, the least square mean±standard error change in diurnal IOP from baseline was -7.2±0.34 mmHg and -7.3±0.34 mmHg with BBFC and BRINZ+BRIM, respectively (between-group difference: 0.1 mmHg [95% CI -0.5, 0.7]). In the BBFC and BRINZ+BRIM groups, 53.3% and 55.0% of patients achieved a diurnal IOP <18 mmHg, and 43.2% and 37.4% of patients, respectively, achieved a mean diurnal IOP reduction >30% from baseline at Month 3. Ocular AEs were reported in 28.7% (BBFC) and 22.5% (BRINZ+BRIM) of patients; conjunctival hyperemia was the most frequent ocular AE (BBFC, 6.4%; BRINZ+BRIM, 6.8%). Non-ocular AEs were reported in 32.4% (BBFC) and 30.4% (BRINZ+BRIM) of patients.
Conclusion: The study findings demonstrate that the efficacy of twice-daily BBFC was non-inferior to BRINZ+BRIM in patients with OAG/OHT. The safety profile of BBFC was similar to that of BRINZ+BRIM.
Keywords: brinzolamide/brimonidine fixed-dose combination; intraocular pressure reduction; ocular hypertension; open-angle glaucoma.
© 2020 Wang et al.
Conflict of interest statement
Meeting presentation: Data included in this manuscript were previously presented at the following congresses: 13th European glaucoma society (EGS) 2018, Florence, Italy; 36th World Ophthalmology Congress (WOC) 2018, Barcelona, Spain. Ningli Wang has received honoraria and personal fees from Novartis. Adeniyi Adewale and Thomas M. Walker are employees of Novartis. Adeniyi Adewale is also a marketer of brinzolamide/brimonidine fixed-dose combination. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Randomized trial of brinzolamide/brimonidine versus brinzolamide plus brimonidine for open-angle glaucoma or ocular hypertension.Adv Ther. 2014 Dec;31(12):1213-27. doi: 10.1007/s12325-014-0168-y. Epub 2014 Nov 28. Adv Ther. 2014. PMID: 25430900 Free PMC article. Clinical Trial.
-
Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension.Ophthalmology. 2014 Dec;121(12):2348-55. doi: 10.1016/j.ophtha.2014.06.022. Epub 2014 Jul 23. Ophthalmology. 2014. PMID: 25064721 Clinical Trial.
-
Maximum Medical Therapy: Brinzolamide/Brimonidine And Travoprost/Timolol Fixed-Dose Combinations In Glaucoma And Ocular Hypertension.Clin Ophthalmol. 2019 Dec 5;13:2411-2419. doi: 10.2147/OPTH.S228777. eCollection 2019. Clin Ophthalmol. 2019. PMID: 31824135 Free PMC article.
-
Combination of brinzolamide and brimonidine for glaucoma and ocular hypertension: critical appraisal and patient focus.Patient Prefer Adherence. 2014 Jun 12;8:853-64. doi: 10.2147/PPA.S53162. eCollection 2014. Patient Prefer Adherence. 2014. PMID: 24966670 Free PMC article. Review.
-
Clinical effectiveness of brinzolamide 1%-brimonidine 0.2% fixed combination for primary open-angle glaucoma and ocular hypertension.Clin Ophthalmol. 2015 Nov 24;9:2201-7. doi: 10.2147/OPTH.S72380. eCollection 2015. Clin Ophthalmol. 2015. PMID: 26648686 Free PMC article. Review.
Cited by
-
Evaluation of the Use of Brinzolamide-Brimonidine Fixed Combination in Maximum Medical Therapy.Turk J Ophthalmol. 2022 Aug 25;52(4):262-269. doi: 10.4274/tjo.galenos.2021.25488. Turk J Ophthalmol. 2022. PMID: 36017234 Free PMC article.
References
-
- Leibowitz HM, Krueger DE, Maunder LR, et al. The framingham eye study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(Suppl):335–610. - PubMed
-
- Blumberg D, Skaat A, Liebmann JM. Emerging risk factors for glaucoma onset and progression. Prog Brain Res. 2015;221:81–101. - PubMed
LinkOut - more resources
Full Text Sources

