Entire ABL1 Gene Deletion Without BCR/ABL1 Rearrangement in a Female Patient with B-Cell Precursor Acute Lymphoblastic Leukemia
- PMID: 32158229
- PMCID: PMC6986541
- DOI: 10.2147/OTT.S238336
Entire ABL1 Gene Deletion Without BCR/ABL1 Rearrangement in a Female Patient with B-Cell Precursor Acute Lymphoblastic Leukemia
Abstract
Acute lymphoblastic leukemia (ALL) is a malignant disease characterized by lymphocytic B-line or T-line cells abnormally proliferating in the bone marrow or extramedullary sites. BCR/ABL1 fusion protein in patients with ALL accounts for acts in 15-30% of B-lineage ALL cases, usually in adolescence. However, entire ABL1 gene deletion without BCR/ABL1 rearrangement is a rare phenomenon in ALL patients. Here we describe the first case of entire ABL1 gene deletion without BCR/ABL1 rearrangement in a female B-ALL patient. Relevant literature is reviewed to explain the association between ABL1 deletion and the pathogenesis/prognosis of this disease. ABL gene deletion can repress the activation of p53 and p73, and disrupt TGF-β signaling pathway to allow malignant cells to invade the normal tissue. The clinical significance of ABL gene deletion needs to be further explored.
Keywords: ABL1 deletion; ALL; acute lymphoblastic leukemia; pathogenesis; prognosis.
© 2020 Jiang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

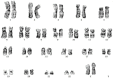




Similar articles
-
ABL1 gene deletion without BCR/ABL1 rearrangement in a young adolescent with precursor B-cell acute lymphoblastic leukemia: clinical study and literature review.Cancer Genet Cytogenet. 2010 Jan 15;196(2):184-8. doi: 10.1016/j.cancergencyto.2009.09.018. Cancer Genet Cytogenet. 2010. PMID: 20082857 Review.
-
High frequency of BTG1 deletions in patients with BCR-ABL1-positive acute leukemia.Cancer Genet. 2014 May;207(5):226-30. doi: 10.1016/j.cancergen.2014.05.003. Epub 2014 May 16. Cancer Genet. 2014. PMID: 24998463
-
BCR-ABL1 induces aberrant splicing of IKAROS and lineage infidelity in pre-B lymphoblastic leukemia cells.Oncogene. 2006 Feb 16;25(7):1118-24. doi: 10.1038/sj.onc.1209133. Oncogene. 2006. PMID: 16205638
-
The BCR-ABL1 kinase bypasses selection for the expression of a pre-B cell receptor in pre-B acute lymphoblastic leukemia cells.J Exp Med. 2004 Mar 1;199(5):673-85. doi: 10.1084/jem.20031637. J Exp Med. 2004. PMID: 14993251 Free PMC article.
-
Laboratory testing in BCR-ABL1-like (Philadelphia-like) B-lymphoblastic leukemia/lymphoma.Am J Hematol. 2018 Jul;93(7):971-977. doi: 10.1002/ajh.25126. Epub 2018 May 15. Am J Hematol. 2018. PMID: 29696694 Review.
Cited by
-
Detection of ABL1 deletion without BCR-ABL rearrangement in ETP-ALL.Hematol Transfus Cell Ther. 2024 Oct-Dec;46(4):462-464. doi: 10.1016/j.htct.2022.01.016. Epub 2022 Mar 25. Hematol Transfus Cell Ther. 2024. PMID: 35365438 Free PMC article. No abstract available.
-
ABL1 kinase as a tumor suppressor in AML1-ETO and NUP98-PMX1 leukemias.Blood Cancer J. 2023 Mar 23;13(1):42. doi: 10.1038/s41408-023-00810-0. Blood Cancer J. 2023. PMID: 36959186 Free PMC article.
References
-
- Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87. - PubMed
-
- Calabrese G, Fantasia D, Di Gianfilippo R, Stuppia L, Di Lorenzo R, Palka G. Fluorescence in situ hybridization analysis of minimal residual disease and the relevance of the der(9) deletion in imatinib-treated patients with chronic myeloid leukemia. Haematologica. 2006;91(7):994–995. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

