Omalizumab for Severe Allergic Asthma Treatment in Italy: A Cost-Effectiveness Analysis from PROXIMA Study
- PMID: 32158289
- PMCID: PMC6986414
- DOI: 10.2147/RMHP.S211321
Omalizumab for Severe Allergic Asthma Treatment in Italy: A Cost-Effectiveness Analysis from PROXIMA Study
Abstract
Introduction: Inadequately controlled severe asthma patients require additional therapy accounting for significant clinical and economic burden. Our analysis aims to determine the cost-effectiveness of omalizumab in the management of severe allergic asthma in Italy based on observational data from the PROXIMA study.
Methods: Observational data on efficacy, healthcare resource utilization and changes in quality of life at 12 months after the initiation of omalizumab were examined to estimate the cost-effectiveness compared to pre-omalizumab period and results were expressed with Incremental Cost-Effectiveness Ratio (ICER). The cost-utility analysis estimated the cost per quality-adjusted life-year (QALY) gained. Direct health costs were assessed from the perspective of the Italian National Health Service (NHS).
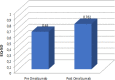
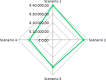
Results: Omalizumab reduced the incidence of exacerbations, number of hospitalizations, physician visits, and improved quality of life after 12 months of treatment. Omalizumab had a greater effectiveness than pre-omalizumab treatment involving 0.132 QALYs gained and led to a €3729 per patient reduction in direct healthcare costs, excluding the add-on treatment cost. Nevertheless, the addition of omalizumab cost led to €7478 increase in total direct costs with respect to pre-omalizumab period. Based on difference in total direct cost and difference in QALY between post and pre-omalizumab period, the ICER was €56,847. According to sensitivity analysis, omalizumab provided a cost-effective use of NHS resources, already at 20% discounted price.
Conclusion: This study offers a real-world evidence of omalizumab effectiveness in Italy. Despite the high acquisition cost of the innovative drug, omalizumab is a sustainable treatment option for patients with uncontrolled severe allergic asthma.
Keywords: PROXIMA study; cost-utility; effectiveness; healthcare costs; omalizumab; severe allergic asthma.
© 2020 Canonica et al.
Conflict of interest statement
GLC, GMB, SDM, CM are employees of S.A.V.E. S.r.l and consultants for Novartis. CP is an employee of Novartis Pharma, Origgio, Italy. GLC has received research and educational grants from Abbott, Amgen, EISAI, Novo Nordisk, Menarini, Otsuka and Jazzpharma. P.S. has been consultant for Novartis. GWC has been speaker & consultant for Novartis. PR reports grants and/or personal fees from Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, ChiesiFarmaceutici, GlaxoSmithKline, Menarini, Mundipharma, Novartis, and Zambon. The authors report no other conflicts of interest in this work.
Figures

References
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2016. Available from: www.ginasthma.org.Accessed December11, 2019.
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. 2019. Available from: www.ginaasthma.org. Accessed December11, 2019.

