Re-defining T-Cell Exhaustion: Subset, Function, and Regulation
- PMID: 32158590
- PMCID: PMC7049579
- DOI: 10.4110/in.2020.20.e2
Re-defining T-Cell Exhaustion: Subset, Function, and Regulation
Abstract
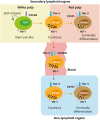
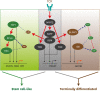
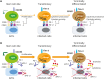
Acute viral infection or vaccination generates highly functional memory CD8 T cells following the Ag resolution. In contrast, persistent antigenic stimulation in chronic viral infection and cancer leads to a state of T-cell dysfunction termed T-cell exhaustion. We and other have recently identified a novel subset of exhausted CD8 T cells that act as stem cells for maintaining virus-specific CD8 T cells in a mouse model of chronic lymphocytic choriomeningitis virus infection. This stem cell-like CD8 T-cell subset has been also observed in both mouse and human tumor models. Most importantly, in both chronic viral infection and tumor models, the proliferative burst of Ag-specific CD8 T cells driven by PD-1-directed immunotherapy comes exclusively from this stem cell-like CD8 T-cell subset. Therefore, a better understanding of the mechanisms how CD8 T-cell subsets are regulated during chronic viral infection and cancer is required to improve the current immunotherapies that restore the function of exhausted CD8 T cells. In this review, we discuss the differentiation of virus-specific CD8 T cells during chronic viral infection, the characteristics and function of CD8 T-cell subsets, and the therapeutic intervention of PD-1-directed immunotherapy in cancer.
Keywords: Immunotherapy; PD-1; Stem cell-like CD8 T-cell subset; T-cell exhaustion.
Copyright © 2020. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures

References
-
- Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, Araki K, Ahmed R. CD8 T cell exhaustion in chronic infection and cancer: opportunities for interventions. Annu Rev Med. 2018;69:301–318. - PubMed
-
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. - PubMed
-
- McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. - PubMed
-
- Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

