Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors
- PMID: 32158597
- PMCID: PMC7049586
- DOI: 10.4110/in.2020.20.e9
Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors
Abstract
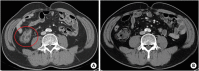
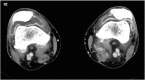
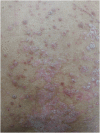
Immune checkpoint inhibitors (ICIs) have been changing the paradigm of cancer treatment. However, immune-related adverse effects (irAEs) have also increased with the exponential increase in the use of ICIs. ICIs can break up the immunologic homeostasis and reduce T-cell tolerance. Therefore, inhibition of immune checkpoint can lead to the activation of autoreactive T-cells, resulting in various irAEs similar to autoimmune diseases. Gastrointestinal toxicity, endocrine toxicity, and dermatologic toxicity are common side effects. Neurotoxicity, cardiotoxicity, and pulmonary toxicity are relatively rare but can be fatal. ICI-related gastrointestinal toxicity, dermatologic toxicity, and hypophysitis are more common with anti- CTLA-4 agents. ICI-related pulmonary toxicity, thyroid dysfunction, and myasthenia gravis are more common with PD-1/PD-L1 inhibitors. Treatment with systemic steroids is the principal strategy against irAEs. The use of immune-modulatory agents should be considered in case of no response to the steroid therapy. Treatment under the supervision of multidisciplinary specialists is also essential, because the symptoms and treatments of irAEs could involve many organs. Thus, this review focuses on the mechanism, clinical presentation, incidence, and treatment of various irAEs.
Keywords: Adverse events; Immune checkpoint inhibitor; Programmed cell death 1.
Copyright © 2020. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures
References
-
- Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. 2018;17:854–855. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

