Environmental Determinants of Malaria Transmission Around the Koka Reservoir in Ethiopia
- PMID: 32159012
- PMCID: PMC7007164
- DOI: 10.1002/2017GH000108
Environmental Determinants of Malaria Transmission Around the Koka Reservoir in Ethiopia
Abstract
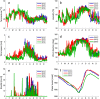
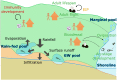
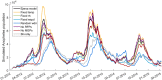
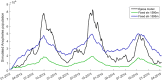
New dam construction is known to exacerbate malaria transmission in Africa as the vectors of malaria-Anopheles mosquitoes-use bodies of water as breeding sites. Precise environmental mechanisms of how reservoirs exacerbate malaria transmission are yet to be identified. Understanding of these mechanisms should lead to a better assessment of the impacts of dam construction and to new prevention strategies. Combining extensive multiyear field surveys around the Koka Reservoir in Ethiopia and rigorous model development and simulation studies, environmental mechanisms of malaria transmission around the reservoir were examined. Most comprehensive and detailed malaria transmission model, Hydrology, Entomology, and Malaria Transmission Simulator, was applied to a village adjacent to the reservoir. Significant contributions to the dynamics of malaria transmission are shaped by wind profile, marginal pools, temperature, and shoreline locations. Wind speed and wind direction influence Anopheles populations and malaria transmission during the major and secondary mosquito seasons. During the secondary mosquito season, a noticeable influence was also attributed to marginal pools. Temperature was found to play an important role, not so much in Anopheles population dynamics, but in malaria transmission dynamics. Change in shoreline locations drives malaria transmission dynamics, with closer shoreline locations to the village making malaria transmission more likely. Identified environmental mechanisms help in predicting malaria transmission seasons and in developing village relocation strategies upon dam construction to minimize the risk of malaria.
Keywords: environmental conditions; malaria transmission; water resource reservoirs.
©2018. The Authors.
Conflict of interest statement
The authors declare no conflicts of interest relevant to this study.
Figures


References
-
- Bharti, A. R. , Patra, K. P. , Chuquiyauri, R. , Kosek, M. , Gilman, R. H. , Llanos‐Cuentas, A. , & Vinetz, J. M. (2007). Short report: Polymerase chain reaction detection of Plasmodium vivax and Plasmodium falciparum DNA from stored serum samples: Implications for retrospective diagnosis of malaria. American Journal of Tropical Medicine and Hygiene, 77(3), 444–446. - PubMed
-
- Bomblies, A. , Duchemin, J.‐B. , & Eltahir, E. A. B. (2008). Hydrology of malaria: Model development and application to a Sahelian village. Water Resources Research, 44, W12445 10.1029/2008WR006917 - DOI
-
- Cano, J. , Descalzo, M. A. , Moreno, M. , Chen, Z. , Nzambo, S. , Bobuakasi, L. , et al. (2006). Spatial variability in the density, distribution and vectorial capacity of anopheline species in a high transmission village (Equatorial Guinea). Malaria Journal, 5, 21 10.1186/1475-2875-5-21 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
