Longitudinal Study of Bacterial Infectious Agents in a Community of Small Mammals in New Mexico
- PMID: 32159462
- PMCID: PMC9536245
- DOI: 10.1089/vbz.2019.2550
Longitudinal Study of Bacterial Infectious Agents in a Community of Small Mammals in New Mexico
Abstract
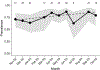
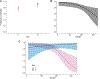
Background and Objectives: Vector-borne bacterial diseases represent a substantial public health burden and rodents have been recognized as important reservoir hosts for many zoonotic pathogens. This study investigates bacterial pathogens in a small mammal community of the southwestern United States of America. Methods: A total of 473 samples from 13 wild rodent and 1 lagomorph species were tested for pathogens of public health significance: Bartonella, Brucella, Yersinia, Borrelia, Rickettsia spp., and Anaplasma phagocytophilum. Results: Three animals were positive for Yersinia pestis, and one Sylvilagus audubonii had a novel Borrelia sp. of the relapsing fever group. No Brucella, Rickettsia, or A. phagocytophilum infections were detected. Bartonella prevalence ranged between 0% and 87.5% by animal species, with 74.3% in the predominant Neotoma micropus and 78% in the second most abundant N. albigula. The mean duration of Bartonella bacteremia in mark-recaptured N. micropus and N. albigula was 4.4 months, ranging from <1 to 18 months, and differed among Bartonella genogroups. Phylogenetic analysis of the Bartonella citrate synthase gene (gltA) revealed 9 genogroups and 13 subgroups. Seven genogroups clustered with known or previously reported Bartonella species and strains while two were distant enough to represent new Bartonella species. We report, for the first time, the detection of Bartonella alsatica in North America in Sylvilagus audubonii and expand the known host range of Bartonella washoensis to include Otospermophilus variegatus. Interpretation and Conclusion: This work broadens our knowledge of the hosts and geographic range of bacterial pathogens that could guide future surveillance efforts and improves our understanding of the dynamics of Bartonella infection in wild small mammals.
Keywords: Bartonella; bacteria; small mammals; vector-borne pathogens; woodrat.
Figures

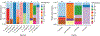

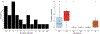

References
-
- Atif FA. Alpha proteobacteria of genus Anaplasma (Rick-ettsiales: Anaplasmataceae): Epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology 2016; 143:659–685. - PubMed
-
- Bai Y, Kosoy M, Calisher C, Cully J Jr., et al. Effects of rodent community diversity and composition on prevalence of an endemic bacterial pathogen-Bartonella. 2009; 10: 3–11.
-
- Bai Y, Kosoy MY, Boonmar S, Sawatwong P, et al. Enrichment culture and molecular identification of diverse Bartonella species in stray dogs. Vet Microbiol 2010; 146:314–319. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

