Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia
- PMID: 32159969
- PMCID: PMC7468846
- DOI: 10.1152/ajplung.00144.2019
Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia
Abstract
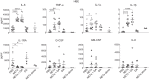
Neutrophil extracellular traps (NETs) provide host defense but can contribute to the pathobiology of diverse human diseases. We sought to determine the extent and mechanism by which NETs contribute to human airway cell inflammation. Primary normal human bronchial epithelial cells (HBEs) grown at air-liquid interface and wild-type (wt)CFBE41o- cells (expressing wtCFTR) were exposed to cell-free NETs from unrelated healthy volunteers for 18 h in vitro. Cytokines were measured in the apical supernatant by Luminex, and the effect on the HBE transcriptome was assessed by RNA sequencing. NETs consistently stimulated IL-8, TNF-α, and IL-1α secretion by HBEs from multiple donors, with variable effects on other cytokines (IL-6, G-CSF, and GM-CSF). Expression of HBE RNAs encoding IL-1 family cytokines, particularly IL-36 subfamily members, was increased in response to NETs. NET exposure in the presence of anakinra [recombinant human IL-1 receptor antagonist (rhIL-1RA)] dampened NET-induced changes in IL-8 and TNF-α proteins as well as IL-36α RNA. rhIL-36RA limited the increase in expression of proinflammatory cytokine RNAs in HBEs exposed to NETs. NETs selectively upregulate an IL-1 family cytokine response in HBEs, which enhances IL-8 production and is limited by rhIL-1RA. The present findings describe a unique mechanism by which NETs may contribute to inflammation in human lung disease in vivo. NET-driven IL-1 signaling may represent a novel target for modulating inflammation in diseases characterized by a substantial NET burden.
Keywords: IL-1; IL-36; IL-8; airway inflammation; neutrophil extracellular trap.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






Similar articles
-
Heterogeneity in Neutrophil Extracellular Traps from Healthy Human Subjects.Int J Mol Sci. 2023 Dec 30;25(1):525. doi: 10.3390/ijms25010525. Int J Mol Sci. 2023. PMID: 38203698 Free PMC article.
-
Neutrophil extracellular traps can serve as platforms for processing and activation of IL-1 family cytokines.FEBS J. 2017 Jun;284(11):1712-1725. doi: 10.1111/febs.14075. Epub 2017 Apr 26. FEBS J. 2017. PMID: 28374518
-
Macrophage-derived IL-1β enhances monosodium urate crystal-triggered NET formation.Inflamm Res. 2017 Mar;66(3):227-237. doi: 10.1007/s00011-016-1008-0. Epub 2016 Nov 16. Inflamm Res. 2017. PMID: 27853847 Free PMC article.
-
NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma.Front Immunol. 2019 Feb 5;10:47. doi: 10.3389/fimmu.2019.00047. eCollection 2019. Front Immunol. 2019. PMID: 30804927 Free PMC article. Review.
-
Computational Methodologies for the in vitro and in situ Quantification of Neutrophil Extracellular Traps.Front Immunol. 2019 Jul 10;10:1562. doi: 10.3389/fimmu.2019.01562. eCollection 2019. Front Immunol. 2019. PMID: 31354718 Free PMC article. Review.
Cited by
-
Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment.Mediators Inflamm. 2020 Jul 16;2020:7527953. doi: 10.1155/2020/7527953. eCollection 2020. Mediators Inflamm. 2020. PMID: 32724296 Free PMC article. Review.
-
Bioinformatics Approach to Identify the Influences of COVID-19 on Ischemic Stroke.Biochem Genet. 2023 Dec;61(6):2222-2241. doi: 10.1007/s10528-023-10366-0. Epub 2023 May 15. Biochem Genet. 2023. PMID: 37184686 Free PMC article.
-
Dipeptidyl peptidase 1 inhibitors and neutrophilic inflammation in bronchiectasis: a narrative review.J Thorac Dis. 2025 Jul 31;17(7):5347-5360. doi: 10.21037/jtd-2025-289. Epub 2025 Jul 8. J Thorac Dis. 2025. PMID: 40809229 Free PMC article. Review.
-
Immunothrombosis in COVID-19: Implications of Neutrophil Extracellular Traps.Biomolecules. 2021 May 6;11(5):694. doi: 10.3390/biom11050694. Biomolecules. 2021. PMID: 34066385 Free PMC article. Review.
-
Reduced serum 25(OH)D is closely related to bronchial mucus plug formation in children with mycoplasma pneumonia: A prospective cohort study.Front Public Health. 2023 Jan 26;11:1099683. doi: 10.3389/fpubh.2023.1099683. eCollection 2023. Front Public Health. 2023. PMID: 36778550 Free PMC article.
References
-
- Anders S, Huber W. Differential Expression of RNA-Seq Data at the Gene Level: the DESeq Package. Heidelberg, Germany: European Molecular Biology Laboratory, 2012.
-
- Apostolidou E, Skendros P, Kambas K, Mitroulis I, Konstantinidis T, Chrysanthopoulou A, Nakos K, Tsironidou V, Koffa M, Boumpas DT, Ritis K. Neutrophil extracellular traps regulate IL-1β-mediated inflammation in familial Mediterranean fever. Ann Rheum Dis 75: 269–277, 2016. doi:10.1136/annrheumdis-2014-205958. - DOI - PubMed
-
- Barrientos L, Marin-Esteban V, de Chaisemartin L, Le-Moal VL, Sandré C, Bianchini E, Nicolas V, Pallardy M, Chollet-Martin S. An improved strategy to recover large fragments of functional human neutrophil extracellular traps. Front Immunol 4: 166, 2013. doi:10.3389/fimmu.2013.00166. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

