Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States
- PMID: 32160149
- PMCID: PMC7258473
- DOI: 10.3201/eid2606.200516
Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States
Abstract
The etiologic agent of an outbreak of pneumonia in Wuhan, China, was identified as severe acute respiratory syndrome coronavirus 2 in January 2020. A patient in the United States was given a diagnosis of infection with this virus by the state of Washington and the US Centers for Disease Control and Prevention on January 20, 2020. We isolated virus from nasopharyngeal and oropharyngeal specimens from this patient and characterized the viral sequence, replication properties, and cell culture tropism. We found that the virus replicates to high titer in Vero-CCL81 cells and Vero E6 cells in the absence of trypsin. We also deposited the virus into 2 virus repositories, making it broadly available to the public health and research communities. We hope that open access to this reagent will expedite development of medical countermeasures.
Keywords: 2019 novel coronavirus disease; COVID-19; PCR; SARS-CoV-2; United States; characterization; coronavirus; isolation; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures

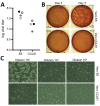
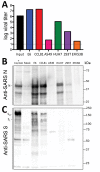
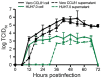
References
-
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. ; Washington State 2019-nCoV Case Investigation Team. Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. 10.1056/NEJMoa2001191 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

