Stromal Cells in the Pathogenesis of Inflammatory Bowel Disease
- PMID: 32160284
- PMCID: PMC7392167
- DOI: 10.1093/ecco-jcc/jjaa009
Stromal Cells in the Pathogenesis of Inflammatory Bowel Disease
Abstract
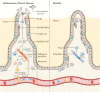
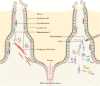
Up till now, research on inflammatory bowel disease [IBD] has mainly been focused on the immune cells present in the gastrointestinal tract. However, recent insights indicate that stromal cells also play an important and significant role in IBD pathogenesis. Stromal cells in the intestines regulate both intestinal epithelial and immune cell homeostasis. Different subsets of stromal cells have been found to play a role in other inflammatory diseases [e.g. rheumatoid arthritis], and these various stromal subsets now appear to carry out also specific functions in the inflamed gut in IBD. Novel potential therapies for IBD utilize, as well as target, these pathogenic stromal cells. Injection of mesenchymal stromal cells [MSCs] into fistula tracts of Crohn's disease patients is already approved and used in clinical settings. In this review we discuss the current knowledge of the role of stromal cells in IBD pathogenesis. We further outline recent attempts to modify the stromal compartment in IBD with agents that target or replace the pathogenic stroma.
Keywords: MSCs; Stromal cells; fibroblasts; inflammatory bowel disease; stroma.
© European Crohn’s and Colitis Organisation (ECCO) 2020.
Figures
References
-
- Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. - PubMed
-
- Reinisch W, Hommes DW, Van Assche G, et al. . A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn’s disease. Gut 2006;55:1138–44. - PMC - PubMed
-
- Schnitzler F, Fidder H, Ferrante M, et al. . Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009;15:1295–301. - PubMed
-
- Rutgeerts P, Van Assche G, Sandborn WJ, et al. . Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012;142:1102–11.e2. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

