Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial
- PMID: 32160294
- PMCID: PMC7229262
- DOI: 10.1182/blood.2020004823
Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial
Abstract
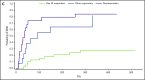
Patients who develop steroid-refractory acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic cell transplantation have poor prognosis, highlighting an unmet therapeutic need. In this open-label phase 2 study (ClinicalTrials.gov identifier: NCT02953678), patients aged at least 12 years with grades II to IV steroid-refractory aGVHD were eligible to receive ruxolitinib orally, starting at 5 mg twice daily plus corticosteroids, until treatment failure, unacceptable toxicity, or death. The primary end point was overall response rate (ORR) at day 28; the key secondary end point was duration of response (DOR) at 6 months. As of 2 July 2018, 71 patients received at least 1 dose of ruxolitinib. Forty-eight of those patients (67.6%) had grade III/IV aGVHD at enrollment. At day 28, 39 patients (54.9%; 95% confidence interval, 42.7%-66.8%) had an overall response, including 19 (26.8%) with complete responses. Best ORR at any time was 73.2% (complete response, 56.3%). Responses were observed across skin (61.1%), upper (45.5%) and lower (46.0%) gastrointestinal tract, and liver (26.7%). Median DOR was 345 days. Overall survival estimate at 6 months was 51.0%. At day 28, 24 (55.8%) of 43 patients receiving ruxolitinib and corticosteroids had a 50% or greater corticosteroid dose reduction from baseline. The most common treatment-emergent adverse events were anemia (64.8%), thrombocytopenia (62.0%), hypokalemia (49.3%), neutropenia (47.9%), and peripheral edema (45.1%). Ruxolitinib produced durable responses and encouraging survival compared with historical data in patients with steroid-refractory aGVHD who otherwise have dismal outcomes. The safety profile was consistent with expectations for ruxolitinib and this patient population.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.J. reports receiving consulting fees from Incyte Corporation, Kadmon, and Genentech and receiving institutional research support from Mallinckrodt and Janssen. M.-A.P. reports receiving institutional research support for clinical trials from Incyte Corporation; honoraria from AbbVie, Bellicum, Bristol-Myers Squibb, Incyte Corporation, Merck, Novartis, Nektar Therapeutics, and Takeda; serving on data and safety monitoring boards for Servier and Medigene; and serving on scientific advisory boards for MolMed and NexImmune. M.A.S. reports receiving personal fees and research grant support from Incyte Corporation, Amgen, AbbVie, Astellas, Pfizer, Sanofi/Genzyme, Takeda, and Merck; receiving personal fees from Partners Therapeutics, FlatIron, and NovoNordisk; and receiving research grant support from Seattle Genetics, PBD Inc, Genentech, Cellect, Fortis Therapeutics, Bristol-Myers Squibb, and Celgene. H.A. reports receiving consulting fees from Incyte Corporation. N.N.S. reports receiving consulting fees from Incyte Corporation; serving on scientific advisory boards for Kite, Juno, and Cellectar; and receiving institutional research support for clinical trials from Miltenyi Biotec. Y.-B.C. reports receiving consulting fees from Incyte Corporation, Takeda, Magenta, and Kiadis and serving on data and safety monitoring boards for Actinium, Equillium, and AbbVie; S.F. reports receiving personal fees and other support for serving on advisory boards and speakers’ bureaus for Amgen, Incyte Corporation, Jazz Pharmaceuticals, Gilead, GlaxoSmithKline, Novartis, Bristol-Myers Squibb, and Pfizer; and receiving personal fees and other support for serving on a speakers’ bureau for Stemline Pharmaceuticals, Janssen Pharmaceuticals, Takeda Pharmaceuticals, and Karyopharm. F.W.D., M.C.A., and C.T. report employment by and stock ownership in Incyte Corporation. M.D.H. reports employment by and stock ownership in Incyte Corporation and has a patent pending for Biomarkers for Graft Versus Host Disease. H.J.K. served on an advisory board and received research funding from Incyte Corporation. L.C.-S. declares no competing financial interests.
Figures




Comment in
-
Ruxolitinib for steroid-resistant acute GVHD.Blood. 2020 May 14;135(20):1721-1722. doi: 10.1182/blood.2020005364. Blood. 2020. PMID: 32407526 No abstract available.
References
-
- D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): 2018 summary slides. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/_layo.... Accessed 8 October 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

