Garcinoic Acid Is a Natural and Selective Agonist of Pregnane X Receptor
- PMID: 32160459
- PMCID: PMC7901650
- DOI: 10.1021/acs.jmedchem.0c00012
Garcinoic Acid Is a Natural and Selective Agonist of Pregnane X Receptor
Abstract
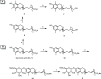
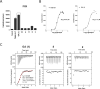
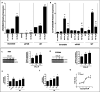
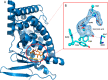
Pregnane X receptor (PXR) is a master xenobiotic-sensing transcription factor and a validated target for immune and inflammatory diseases. The identification of chemical probes to investigate the therapeutic relevance of the receptor is still highly desired. In fact, currently available PXR ligands are not highly selective and can exhibit toxicity and/or potential off-target effects. In this study, we have identified garcinoic acid as a selective and efficient PXR agonist. The properties of this natural molecule as a specific PXR agonist were demonstrated by the screening on a panel of nuclear receptors, the assessment of the physical and thermodynamic binding affinity, and the determination of the PXR-garcinoic acid complex crystal structure. Cytotoxicity, transcriptional, and functional properties were investigated in human liver cells, and compound activity and target engagement were confirmed in vivo in mouse liver and gut tissue. In conclusion, garcinoic acid is a selective natural agonist of PXR and a promising lead compound toward the development of new PXR-regulating modulators.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.G. and R.P. are cofounders of TES Pharma.
Figures


References
-
- Kandel B. A.; Thomas M.; Winter S.; Seehofer D.; Burk O.; Schwab M.; Zanger U. M. Genomewide comparison of the inducible transcriptomes of nuclear receptors CAR, PXR and PPARalpha in primary human hepatocytes. Biochim. Biophys. Acta, Gene Regul. Mech. 2016, 1859, 1218–1227. 10.1016/j.bbagrm.2016.03.007. - DOI - PubMed
-
- Kliewer S. A.; Moore J. T.; Wade L.; Staudinger J. L.; Watson M. A.; Jones S. A.; McKee D. D.; Oliver B. B.; Willson T. M.; Zetterström R. H.; Perlmann T.; Lehmann J. M. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998, 92, 73–82. 10.1016/S0092-8674(00)80900-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Medical
Molecular Biology Databases

