Stepwise Promoter Melting by Bacterial RNA Polymerase
- PMID: 32160514
- PMCID: PMC7166197
- DOI: 10.1016/j.molcel.2020.02.017
Stepwise Promoter Melting by Bacterial RNA Polymerase
Abstract
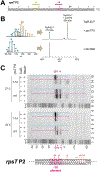
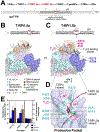
Transcription initiation requires formation of the open promoter complex (RPo). To generate RPo, RNA polymerase (RNAP) unwinds the DNA duplex to form the transcription bubble and loads the DNA into the RNAP active site. RPo formation is a multi-step process with transient intermediates of unknown structure. We use single-particle cryoelectron microscopy to visualize seven intermediates containing Escherichia coli RNAP with the transcription factor TraR en route to forming RPo. The structures span the RPo formation pathway from initial recognition of the duplex promoter in a closed complex to the final RPo. The structures and supporting biochemical data define RNAP and promoter DNA conformational changes that delineate steps on the pathway, including previously undetected transient promoter-RNAP interactions that contribute to populating the intermediates but do not occur in RPo. Our work provides a structural basis for understanding RPo formation and its regulation, a major checkpoint in gene expression throughout evolution.
Keywords: Conformational change; Cryoelectron microscopy; DNA; Open promoter complex formation; Promoter DNA; RNA polymerase; TraR; Transcription initiation.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



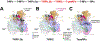
Similar articles
-
Real-Time Single-Molecule Studies of RNA Polymerase-Promoter Open Complex Formation Reveal Substantial Heterogeneity Along the Promoter-Opening Pathway.J Mol Biol. 2022 Jan 30;434(2):167383. doi: 10.1016/j.jmb.2021.167383. Epub 2021 Dec 1. J Mol Biol. 2022. PMID: 34863780 Free PMC article.
-
Structures of RNA Polymerase Closed and Intermediate Complexes Reveal Mechanisms of DNA Opening and Transcription Initiation.Mol Cell. 2017 Jul 6;67(1):106-116.e4. doi: 10.1016/j.molcel.2017.05.010. Epub 2017 Jun 1. Mol Cell. 2017. PMID: 28579332 Free PMC article.
-
Cryo-EM structure of Escherichia coli σ70 RNA polymerase and promoter DNA complex revealed a role of σ non-conserved region during the open complex formation.J Biol Chem. 2018 May 11;293(19):7367-7375. doi: 10.1074/jbc.RA118.002161. Epub 2018 Mar 26. J Biol Chem. 2018. PMID: 29581236 Free PMC article.
-
How to switch the motor on: RNA polymerase initiation steps at the single-molecule level.Protein Sci. 2017 Jul;26(7):1303-1313. doi: 10.1002/pro.3183. Epub 2017 May 12. Protein Sci. 2017. PMID: 28470684 Free PMC article. Review.
-
Initial events in bacterial transcription initiation.Biomolecules. 2015 May 27;5(2):1035-62. doi: 10.3390/biom5021035. Biomolecules. 2015. PMID: 26023916 Free PMC article. Review.
Cited by
-
Approaches to Heterogeneity in Native Mass Spectrometry.Chem Rev. 2022 Apr 27;122(8):7909-7951. doi: 10.1021/acs.chemrev.1c00696. Epub 2021 Sep 1. Chem Rev. 2022. PMID: 34470219 Free PMC article. Review.
-
Early intermediates in bacterial RNA polymerase promoter melting visualized by time-resolved cryo-electron microscopy.bioRxiv [Preprint]. 2024 Mar 14:2024.03.13.584744. doi: 10.1101/2024.03.13.584744. bioRxiv. 2024. Update in: Nat Struct Mol Biol. 2024 Nov;31(11):1778-1788. doi: 10.1038/s41594-024-01349-9. PMID: 38559232 Free PMC article. Updated. Preprint.
-
Structural basis of transcription activation by Rob, a pleiotropic AraC/XylS family regulator.Nucleic Acids Res. 2022 Jun 10;50(10):5974-5987. doi: 10.1093/nar/gkac433. Nucleic Acids Res. 2022. PMID: 35641097 Free PMC article.
-
Structural basis of transcriptional activation by the Mycobacterium tuberculosis intrinsic antibiotic-resistance transcription factor WhiB7.Mol Cell. 2021 Jul 15;81(14):2875-2886.e5. doi: 10.1016/j.molcel.2021.05.017. Epub 2021 Jun 24. Mol Cell. 2021. PMID: 34171296 Free PMC article.
-
Diverse and unified mechanisms of transcription initiation in bacteria.Nat Rev Microbiol. 2021 Feb;19(2):95-109. doi: 10.1038/s41579-020-00450-2. Epub 2020 Oct 29. Nat Rev Microbiol. 2021. PMID: 33122819 Free PMC article. Review.
References
-
- Abascal-Palacios G, Ramsay EP, Beuron F, Morris E, and Vannini A (2018). Structural basis of RNA polymerase III transcription initiation. Nature 553, 301–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

