MERTK-Dependent Ensheathment of Photoreceptor Outer Segments by Human Pluripotent Stem Cell-Derived Retinal Pigment Epithelium
- PMID: 32160519
- PMCID: PMC7066375
- DOI: 10.1016/j.stemcr.2020.02.004
MERTK-Dependent Ensheathment of Photoreceptor Outer Segments by Human Pluripotent Stem Cell-Derived Retinal Pigment Epithelium
Abstract
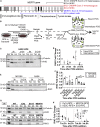
Maintenance of a healthy photoreceptor-retinal pigment epithelium (RPE) interface is essential for vision. At the center of this interface, apical membrane protrusions stemming from the RPE ensheath photoreceptor outer segments (POS), and are possibly involved in the recycling of POS through phagocytosis. The molecules that regulate POS ensheathment and its relationship to phagocytosis remain to be deciphered. By means of ultrastructural analysis, we revealed that Mer receptor tyrosine kinase (MERTK) ligands, GAS6 and PROS1, rather than αVβ5 integrin receptor ligands, triggered POS ensheathment by human embryonic stem cell (hESC)-derived RPE. Furthermore, we found that ensheathment is required for POS fragmentation before internalization. Consistently, POS ensheathment, fragmentation, and internalization were abolished in MERTK mutant RPE, and rescue of MERTK expression in retinitis pigmentosa (RP38) patient RPE counteracted these defects. Our results suggest that loss of ensheathment due to MERTK dysfunction might contribute to vision impairment in RP38 patients.
Keywords: GAS6; MERTK; MFGE8; PROS1; ensheathment; human embryonic stem cells; human pluripotent stem cells; phagocytosis; photoreceptor outer segments; retinal pigment epithelium.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Besharse J.C., Hollyfield J.G., Rayborn M.E. Photoreceptor outer segments: accelerated membrane renewal in rods after exposure to light. Science. 1977;196:536–538. - PubMed
-
- Carr A.-J., Vugler A., Lawrence J., Chen L.L., Ahmado A., Chen F.K., Semo M., Gias C., da Cruz L., Moore H.D. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol. Vis. 2009;15:283–295. - PMC - PubMed
-
- Duncan J.L., LaVail M.M., Yasumura D., Matthes M.T., Yang H., Trautmann N., Chappelow A.V., Feng W., Earp H.S., Matsushima G.K. An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest. Ophthalmol. Vis. Sci. 2002;44:826–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

