Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity
- PMID: 32160525
- PMCID: PMC7469474
- DOI: 10.1016/j.immuni.2020.02.005
Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity
Abstract
Helminths, allergens, and certain protists induce type 2 immune responses, but the underlying mechanisms of immune activation remain poorly understood. In the small intestine, chemosensing by epithelial tuft cells results in the activation of group 2 innate lymphoid cells (ILC2s), which subsequently drive increased tuft cell frequency. This feedforward circuit is essential for intestinal remodeling and helminth clearance. ILC2 activation requires tuft-cell-derived interleukin-25 (IL-25), but whether additional signals regulate the circuit is unclear. Here, we show that tuft cells secrete cysteinyl leukotrienes (cysLTs) to rapidly activate type 2 immunity following chemosensing of helminth infection. CysLTs cooperate with IL-25 to activate ILC2s, and tuft-cell-specific ablation of leukotriene synthesis attenuates type 2 immunity and delays helminth clearance. Conversely, cysLTs are dispensable for the tuft cell response induced by intestinal protists. Our findings identify an additional tuft cell effector function and suggest context-specific regulation of tuft-ILC2 circuits within the small intestine.
Keywords: Heligmosomoides polygyrus; ILC2; Nippostrongylus brasiliensis; brush cell; helminth; intestine; leukotriene; protist; succinate; tuft cell.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
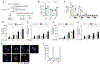

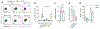

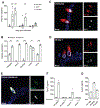


Comment in
-
A Tuft Act to Follow: Leukotrienes Take the Stage in Anti-worm Immunity.Immunity. 2020 Mar 17;52(3):426-428. doi: 10.1016/j.immuni.2020.02.011. Immunity. 2020. PMID: 32187512
References
-
- Bäck M, Dahlén S-E, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, and Rovati GE (2011). International Union of Basic and Clinical Pharmacology. LXXXIV: Leukotriene Receptor Nomenclature, Distribution, and Pathophysiological Functions. Pharmacol. Rev 63, 539–584. - PubMed
-
- Barker N (2013). Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol 15, 19. - PubMed
-
- Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, et al. (2013). IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol 132, 933–941. - PubMed
-
- Beller TC, Maekawa A, Friend DS, Austen KF, and Kanaoka Y (2004). Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J. Biol. Chem 279, 46129–46134. - PubMed
-
- Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, and Damak S (2008). Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J. Comp. Neurol 509, 514–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

