Individual Limb Muscle Bundles Are Formed through Progressive Steps Orchestrated by Adjacent Connective Tissue Cells during Primary Myogenesis
- PMID: 32160556
- PMCID: PMC7068676
- DOI: 10.1016/j.celrep.2020.02.037
Individual Limb Muscle Bundles Are Formed through Progressive Steps Orchestrated by Adjacent Connective Tissue Cells during Primary Myogenesis
Abstract
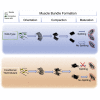
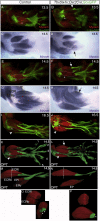
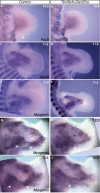
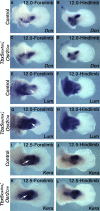
Although the factors regulating muscle cell differentiation are well described, we know very little about how differentiating muscle fibers are organized into individual muscle tissue bundles. Disruption of these processes leads to muscle hypoplasia or dysplasia, and replicating these events is vital in tissue engineering approaches. We describe the progressive cellular events that orchestrate the formation of individual limb muscle bundles and directly demonstrate the role of the connective tissue cells that surround muscle precursors in controlling these events. We show how disruption of gene activity within or genetic ablation of connective tissue cells impacts muscle precursors causing disruption of muscle bundle formation and subsequent muscle dysplasia and hypoplasia. We identify several markers of the populations of connective tissue cells that surround muscle precursors and provide a model for how matrix-modifying proteoglycans secreted by these cells may influence muscle bundle formation by effects on the local extracellular matrix (ECM) environment.
Keywords: HOS; Holt-Oram syndrome; Tbx5; irregular connective tissue; muscle; muscle connective tissue; tissue morphogenesis.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




References
-
- Ameye L., Aria D., Jepsen K., Oldberg A., Xu T., Young M.F. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. - PubMed
-
- Baldock R., Bard J., Brune R., Hill B., Kaufman M., Opstad K., Smith D., Stark M., Waterhouse A., Yang Y., Davidson D. The Edinburgh Mouse Atlas: using the CD. Brief. Bioinform. 2001;2:159–169. - PubMed
-
- Basson C.T., Bachinsky D.R., Lin R.C., Levi T., Elkins J.A., Soults J., Grayzel D., Kroumpouzou E., Traill T.A., Leblanc-Straceski J. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat. Genet. 1997;15:30–35. - PubMed
-
- Brandan E., Gutierrez J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013;32:289–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

