Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration
- PMID: 32160558
- PMCID: PMC7091476
- DOI: 10.1016/j.celrep.2020.02.067
Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration
Abstract
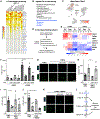
Muscle regeneration relies on the regulation of muscle stem cells (MuSCs) through paracrine signaling interactions. We analyzed muscle regeneration in mice using single-cell RNA sequencing (scRNA-seq) and generated over 34,000 single-cell transcriptomes spanning four time-points. We identified 15 distinct cell types including heterogenous populations of muscle stem and progenitor cells. We resolved a hierarchical map of these myogenic cells by trajectory inference and observed stage-specific regulatory programs within this continuum. Through ligand-receptor interaction analysis, we identified over 100 candidate regeneration-associated paracrine communication pairs between MuSCs and non-myogenic cells. We show that myogenic stem/progenitor cells exhibit heterogeneous expression of multiple Syndecan proteins in cycling myogenic cells, suggesting that Syndecans may coordinate myogenic fate regulation. We performed ligand stimulation in vitro and confirmed that three paracrine factors (FGF2, TGFβ1, and RSPO3) regulate myogenic cell proliferation in a Syndecan-dependent manner. Our study provides a scRNA-seq reference resource to investigate cell communication interactions in muscle regeneration.
Keywords: ligand receptor interaction; muscle stem cells; myogenic differentiation; single-cell RNA-sequencing; skeletal muscle regeneration; syndecans.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no conflicts of interest.
Figures


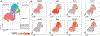


References
-
- Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, and Newell EW (2018). Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol 37, 34–44(2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

