Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality
- PMID: 32160663
- PMCID: PMC7317648
- DOI: 10.1056/NEJMoa1912035
Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality
Abstract
Background: More information is needed about the long-term effects of low-dose aspirin (≤160 mg) on incident hepatocellular carcinoma, liver-related mortality, and gastrointestinal bleeding in persons with chronic hepatitis B or hepatitis C virus infection.
Methods: Using nationwide Swedish registries, we identified all adults who received a diagnosis of chronic hepatitis B or hepatitis C from 2005 through 2015 and who did not have a history of aspirin use (50,275 patients). Patients who were starting to take low-dose aspirin (14,205 patients) were identified by their first filled prescriptions for 90 or more consecutive doses of aspirin. We constructed a propensity score and applied inverse probability of treatment weighting to balance baseline characteristics between groups. Using Cox proportional-hazards regression modeling, we estimated the risk of hepatocellular carcinoma and liver-related mortality, accounting for competing events.
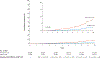
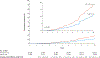
Results: With a median of 7.9 years of follow-up, the estimated cumulative incidence of hepatocellular carcinoma was 4.0% among aspirin users and 8.3% among nonusers of aspirin (difference, -4.3 percentage points; 95% confidence interval [CI], -5.0 to -3.6; adjusted hazard ratio, 0.69; 95% CI, 0.62 to 0.76). This inverse association appeared to be duration-dependent; as compared with short-term use (3 months to <1 year), the adjusted hazard ratios were 0.90 (95% CI, 0.76 to 1.06) for 1 to less than 3 years of use, 0.66 (95% CI, 0.56 to 0.78) for 3 to less than 5 years of use, and 0.57 (95% CI, 0.42 to 0.70) for 5 or more years of use. Ten-year liver-related mortality was 11.0% among aspirin users and 17.9% among nonusers (difference, -6.9 percentage points [95% CI, -8.1 to -5.7]; adjusted hazard ratio, 0.73 [95% CI, 0.67 to 0.81]). However, the 10-year risk of gastrointestinal bleeding did not differ significantly between users and nonusers of aspirin (7.8% and 6.9%, respectively; difference, 0.9 percentage points; 95% CI, -0.6 to 2.4).
Conclusions: In a nationwide study of patients with chronic viral hepatitis in Sweden, use of low-dose aspirin was associated with a significantly lower risk of hepatocellular carcinoma and lower liver-related mortality than no use of aspirin, without a significantly higher risk of gastrointestinal bleeding. (Funded by the National Institutes of Health and others.).
Copyright © 2020 Massachusetts Medical Society.
Figures
Comment in
-
Association between Aspirin and Hepatocellular Carcinoma.N Engl J Med. 2020 Jun 18;382(25):2480-2481. doi: 10.1056/NEJMc2009497. N Engl J Med. 2020. PMID: 32558476 No abstract available.
-
Association between Aspirin and Hepatocellular Carcinoma.N Engl J Med. 2020 Jun 18;382(25):2481. doi: 10.1056/NEJMc2009497. N Engl J Med. 2020. PMID: 32558477 No abstract available.
-
Ein wenig ASS beugt Leberkrebs vor : Hepatitis B und C -- Autor: G. Klose.MMW Fortschr Med. 2020 Oct;162(18):30. doi: 10.1007/s15006-020-4483-8. MMW Fortschr Med. 2020. PMID: 33074498 German. No abstract available.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. - PubMed
-
- Kern MA, Schubert D, Sahi D, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology 2002; 36:8 85–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
