Sickle Cell Anemia Mediates Carotid Artery Expansive Remodeling That Can Be Prevented by Inhibition of JNK (c-Jun N-Terminal Kinase)
- PMID: 32160775
- PMCID: PMC7224328
- DOI: 10.1161/ATVBAHA.120.314045
Sickle Cell Anemia Mediates Carotid Artery Expansive Remodeling That Can Be Prevented by Inhibition of JNK (c-Jun N-Terminal Kinase)
Abstract
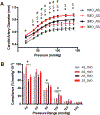
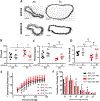
Objective: Sickle cell anemia (SCA) causes chronic inflammation and multiorgan damage. Less understood are the arterial complications, most evident by increased strokes among children. Proteolytic mechanisms, biomechanical consequences, and pharmaceutical inhibitory strategies were studied in a mouse model to provide a platform for mechanistic and intervention studies of large artery damage due to sickle cell disease. Approach and Results: Townes humanized transgenic mouse model of SCA was used to test the hypothesis that elastic lamina and structural damage in carotid arteries increased with age and was accelerated in mice homozygous for SCA (sickle cell anemia homozygous genotype [SS]) due to inflammatory signaling pathways activating proteolytic enzymes. Elastic lamina fragmentation observed by 1 month in SS mice compared with heterozygous littermate controls (sickle cell trait heterozygous genotype [AS]). Positive immunostaining for cathepsin K, a powerful collagenase and elastase, confirmed accelerated proteolytic activity in SS carotids. Larger cross-sectional areas were quantified by magnetic resonance angiography and increased arterial compliance in SS carotids were also measured. Inhibiting JNK (c-jun N-terminal kinase) signaling with SP600125 significantly reduced cathepsin K expression, elastin fragmentation, and carotid artery perimeters in SS mice. By 5 months of age, continued medial thinning and collagen degradation was mitigated by treatment of SS mice with JNK inhibitor.
Conclusions: Arterial remodeling due to SCA is mediated by JNK signaling, cathepsin proteolytic upregulation, and degradation of elastin and collagen. Demonstration in Townes mice establishes their utility for mechanistic studies of arterial vasculopathy, related complications, and therapeutic interventions for large artery damage due to SCA.
Keywords: carotid arteries; collagen; compliance; elastin; hematology.
Figures



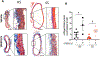
References
-
- Francis RB. Large-vessel occlusion in sickle cell disease: Pathogenesis, clinical consequences, and therapeutic implications. Med Hypotheses. 1991;35:88–95. - PubMed
-
- Adams R, McKie V, Nichols F, Carl E, Zhang DL, McKie K, Figueroa R, Litaker M, Thompson W, Hess D. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–610. - PubMed
-
- Powars D, Wilson B, Imbus C, Pegelow C, Allen J. The natural history of stroke in sickle cell disease. Am J Med. 1978;65:461–471. - PubMed
-
- Merkel KH, Ginsberg PL, Parker JC, Post MJ. Cerebrovascular disease in sickle cell anemia: A clinical, pathological and radiological correlation. Stroke. 1978;9:45–52. - PubMed
-
- Switzer JA, Hess DC, Nichols FT, Adams RJ. Pathophysiology and treatment of stroke in sickle-cell disease: Present and future. Lancet neurology. 2006;5:501–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

