Atherogenic Lipoprotein(a) Increases Vascular Glycolysis, Thereby Facilitating Inflammation and Leukocyte Extravasation
- PMID: 32160811
- PMCID: PMC7208285
- DOI: 10.1161/CIRCRESAHA.119.316206
Atherogenic Lipoprotein(a) Increases Vascular Glycolysis, Thereby Facilitating Inflammation and Leukocyte Extravasation
Abstract
Rationale: Patients with elevated levels of lipoprotein(a) [Lp(a)] are hallmarked by increased metabolic activity in the arterial wall on positron emission tomography/computed tomography, indicative of a proinflammatory state.
Objective: We hypothesized that Lp(a) induces endothelial cell inflammation by rewiring endothelial metabolism.
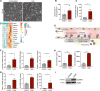
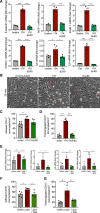
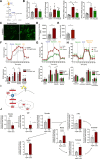
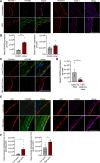
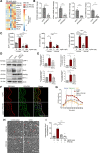
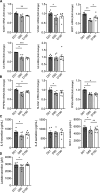
Methods and results: We evaluated the impact of Lp(a) on the endothelium and describe that Lp(a), through its oxidized phospholipid content, activates arterial endothelial cells, facilitating increased transendothelial migration of monocytes. Transcriptome analysis of Lp(a)-stimulated human arterial endothelial cells revealed upregulation of inflammatory pathways comprising monocyte adhesion and migration, coinciding with increased 6-phophofructo-2-kinase/fructose-2,6-biphosphatase (PFKFB)-3-mediated glycolysis. ICAM (intercellular adhesion molecule)-1 and PFKFB3 were also found to be upregulated in carotid plaques of patients with elevated levels of Lp(a). Inhibition of PFKFB3 abolished the inflammatory signature with concomitant attenuation of transendothelial migration.
Conclusions: Collectively, our findings show that Lp(a) activates the endothelium by enhancing PFKFB3-mediated glycolysis, leading to a proadhesive state, which can be reversed by inhibition of glycolysis. These findings pave the way for therapeutic agents targeting metabolism aimed at reducing inflammation in patients with cardiovascular disease.
Keywords: endothelial cell; glycolysis; inflammation; lipoprotein(a); metabolism.
Figures
Comment in
-
Lipoprotein(a) Gets Worse.Circ Res. 2020 May 8;126(10):1360-1362. doi: 10.1161/CIRCRESAHA.120.316980. Epub 2020 May 7. Circ Res. 2020. PMID: 32379575 Free PMC article. No abstract available.
References
-
- Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Hörkkö S, Krauss RM, Chapman MJ, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. - PubMed
-
- Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042. - PubMed
-
- van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, et al. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

