Successful recruitment of a multi-site international randomized placebo-controlled trial in people with HIV with attention to diversity of race and ethnicity: critical role of central coordination
- PMID: 32160827
- PMCID: PMC7664829
- DOI: 10.1080/25787489.2020.1733794
Successful recruitment of a multi-site international randomized placebo-controlled trial in people with HIV with attention to diversity of race and ethnicity: critical role of central coordination
Abstract
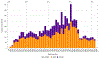
Background: The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) is a multicenter, randomized, placebo-controlled trial, designed to test whether a statin medication can prevent cardiovascular disease in people with HIV. REPRIEVE recently completed enrollment of 7557 participants at over 100 clinical sites globally. Participant groups of focus were women, and racial and ethnic minorities.Objective: To describe recruitment methods and strategies developed by the REPRIEVE Clinical Coordinating Center (CCC) and share best practices learned from the recruitment process.Methods: Enrollment targets were agreed upon with the primary funder, the National Heart, Lung, and Blood Institute (NHLBI) and were milestone driven. Milestones included number of sites activated, number of participants enrolled within specific time frames, and proportion of women and minorities enrolled. Strategies to achieve these milestones included structured interviews with site-designated REPRIEVE Recruitment Champions to develop best practices, development of a multimedia campaign, and site level recruitment support.Results: Recruitment initiated March, 2015 and completed March, 2019. The final accrual target was 7500 participants over 48 months. The trial met this target within the time specified. Overall, 10,613 screens were completed, 48% of participants enrolled from sites outside of North America, 32% were female, 44% were Black or African American, and 25% were Hispanic or Latino.Conclusions: REPRIEVE met its overall projected recruitment goal by using multiple, simultaneous strategies to specifically target a diverse population including minority subgroups. REPRIEVE benefited from the development of recruitment strategies with clear targets and communication of accrual targets to study teams.
Keywords: Clinical Coordinating Center; HIV; REPRIEVE; cardiovascular disease; primary prevention; recruitment.
Figures


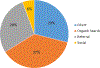
References
-
- Treweek S, Pitkethly M, Cook J, Kjeldstrom M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010. April 14(4):MR000013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
