Obesity: sex and sympathetics
- PMID: 32160920
- PMCID: PMC7065372
- DOI: 10.1186/s13293-020-00286-8
Obesity: sex and sympathetics
Abstract
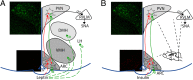
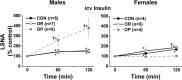
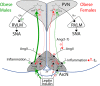
Obesity increases sympathetic nerve activity (SNA) in men, but not women. Here, we review current evidence suggesting that sexually dimorphic sympathoexcitatory responses to leptin and insulin may contribute. More specifically, while insulin increases SNA similarly in lean males and females, this response is markedly amplified in obese males, but is abolished in obese females. In lean female rats, leptin increases a subset of sympathetic nerves only during the high estrogen proestrus reproductive phase; thus, in obese females, because reproductive cycling can become impaired, the sporadic nature of leptin-induced sympathoexcitaton could minimize its action, despite elevated leptin levels. In contrast, in males, obesity preserves or enhances the central sympathoexcitatory response to leptin, and current evidence favors leptin's contribution to the well-established increases in SNA induced by obesity in men. Leptin and insulin increase SNA via receptor binding in the hypothalamic arcuate nucleus and a neuropathway that includes arcuate neuropeptide Y (NPY) and proopiomelanocortin (POMC) projections to the paraventricular nucleus. These metabolic hormones normally suppress sympathoinhibitory NPY neurons and activate sympathoexcitatory POMC neurons. However, obesity appears to alter the ongoing activity and responsiveness of arcuate NPY and POMC neurons in a sexually dimorphic way, such that SNA increases in males but not females. We propose hypotheses to explain these sex differences and suggest areas of future research.
Keywords: Arcuate nucleus; Hypertension; Insulin; Leptin; Neuropeptide Y; Paraventricular nucleus; Sympathetic nerve activity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

