Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis
- PMID: 32161042
- PMCID: PMC7190038
- DOI: 10.1136/bmj.m540
Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis
Abstract
Objective: To identify, appraise, and synthesise the best available evidence on the efficacy of perioperative interventions to reduce postoperative pulmonary complications (PPCs) in adult patients undergoing non-cardiac surgery.
Design: Systematic review and meta-analysis of randomised controlled trials.
Data sources: Medline, Embase, CINHAL, and CENTRAL from January 1990 to December 2017.
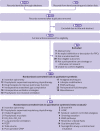
Eligibility criteria: Randomised controlled trials investigating short term, protocolised medical interventions conducted before, during, or after non-cardiac surgery were included. Trials with clinical diagnostic criteria for PPC outcomes were included. Studies of surgical technique or physiological or biochemical outcomes were excluded.
Data extraction and synthesis: Reviewers independently identified studies, extracted data, and assessed the quality of evidence. Meta-analyses were conducted to calculate risk ratios with 95% confidence intervals. Quality of evidence was summarised in accordance with GRADE methods. The primary outcome was the incidence of PPCs. Secondary outcomes were respiratory infection, atelectasis, length of hospital stay, and mortality. Trial sequential analysis was used to investigate the reliability and conclusiveness of available evidence. Adverse effects of interventions were not measured or compared.
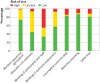
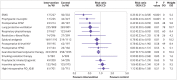
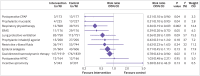
Results: 117 trials enrolled 21 940 participants, investigating 11 categories of intervention. 95 randomised controlled trials enrolling 18 062 participants were included in meta-analysis; 22 trials were excluded from meta-analysis because the interventions were not sufficiently similar to be pooled. No high quality evidence was found for interventions to reduce the primary outcome (incidence of PPCs). Seven interventions had low or moderate quality evidence with confidence intervals indicating a probable reduction in PPCs: enhanced recovery pathways (risk ratio 0.35, 95% confidence interval 0.21 to 0.58), prophylactic mucolytics (0.40, 0.23 to 0.67), postoperative continuous positive airway pressure ventilation (0.49, 0.24 to 0.99), lung protective intraoperative ventilation (0.52, 0.30 to 0.88), prophylactic respiratory physiotherapy (0.55, 0.32 to 0.93), epidural analgesia (0.77, 0.65 to 0.92), and goal directed haemodynamic therapy (0.87, 0.77 to 0.98). Moderate quality evidence showed no benefit for incentive spirometry in preventing PPCs. Trial sequential analysis adjustment confidently supported a relative risk reduction of 25% in PPCs for prophylactic respiratory physiotherapy, epidural analgesia, enhanced recovery pathways, and goal directed haemodynamic therapies. Insufficient data were available to support or refute equivalent relative risk reductions for other interventions.
Conclusions: Predominantly low quality evidence favours multiple perioperative PPC reduction strategies. Clinicians may choose to reassess their perioperative care pathways, but the results indicate that new trials with a low risk of bias are needed to obtain conclusive evidence of efficacy for many of these interventions.
Study registration: Prospero CRD42016035662.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Jammer I, Wickboldt N, Sander M, et al. European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM) European Society of Anaesthesiology. European Society of Intensive Care Medicine Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015;32:88-105. 10.1097/EJA.0000000000000118 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
