Skeletal stem cells: insights into maintaining and regenerating the skeleton
- PMID: 32161063
- PMCID: PMC7075071
- DOI: 10.1242/dev.179325
Skeletal stem cells: insights into maintaining and regenerating the skeleton
Abstract
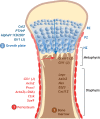
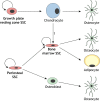
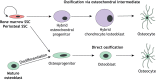
Skeletal stem cells (SSCs) generate the progenitors needed for growth, maintenance and repair of the skeleton. Historically, SSCs have been defined as bone marrow-derived cells with inconsistent characteristics. However, recent in vivo tracking experiments have revealed the presence of SSCs not only within the bone marrow but also within the periosteum and growth plate reserve zone. These studies show that SSCs are highly heterogeneous with regard to lineage potential. It has also been revealed that, during digit tip regeneration and in some non-mammalian vertebrates, the dedifferentiation of osteoblasts may contribute to skeletal regeneration. Here, we examine how these research findings have furthered our understanding of the diversity and plasticity of SSCs that mediate skeletal maintenance and repair.
Keywords: Bone marrow; Growth plate; Periosteum; Skeletal repair; Skeleton; Stem cells.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
-
- Akiyama H., Kim J.-E., Nakashima K., Balmes G., Iwai N., Deng J. M., Zhang Z., Martin J. F., Behringer R. R., Nakamura T. et al. (2005). Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl. Acad. Sci. USA 102, 14665-14670. 10.1073/pnas.0504750102 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

