Repression of sphingosine kinase (SK)-interacting protein (SKIP) in acute myeloid leukemia diminishes SK activity and its re-expression restores SK function
- PMID: 32161116
- PMCID: PMC7170527
- DOI: 10.1074/jbc.RA119.010467
Repression of sphingosine kinase (SK)-interacting protein (SKIP) in acute myeloid leukemia diminishes SK activity and its re-expression restores SK function
Abstract
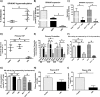
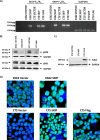
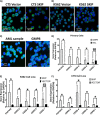
Previous studies have shown that sphingosine kinase interacting protein (SKIP) inhibits sphingosine kinase (SK) function in fibroblasts. SK phosphorylates sphingosine producing the potent signaling molecule sphingosine-1-phosphate (S1P). SKIP gene (SPHKAP) expression is silenced by hypermethylation of its promoter in acute myeloid leukemia (AML). However, why SKIP activity is silenced in primary AML cells is unclear. Here, we investigated the consequences of SKIP down-regulation in AML primary cells and the effects of SKIP re-expression in leukemic cell lines. Using targeted ultra-HPLC-tandem MS (UPLC-MS/MS), we measured sphingolipids (including S1P and ceramides) in AML and control cells. Primary AML cells had significantly lower SK activity and intracellular S1P concentrations than control cells, and SKIP-transfected leukemia cell lines exhibited increased SK activity. These findings show that SKIP re-expression enhances SK activity in leukemia cells. Furthermore, other bioactive sphingolipids such as ceramide were also down-regulated in primary AML cells. Of note, SKIP re-expression in leukemia cells increased ceramide levels 2-fold, inactivated the key signaling protein extracellular signal-regulated kinase, and increased apoptosis following serum deprivation or chemotherapy. These results indicate that SKIP down-regulation in AML reduces SK activity and ceramide levels, an effect that ultimately inhibits apoptosis in leukemia cells. The findings of our study contrast with previous results indicating that SKIP inhibits SK function in fibroblasts and therefore challenge the notion that SKIP always inhibits SK activity.
Keywords: Acute myeloid leukemia; Sphingosine kinase (SphK); cell signaling; ceramide; chemotherapy; cytarabine; hypermethylation; leukemia; lipid metabolism; sphingolipid; sphingosine kinase interacting protein; sphingosine-1-phosphate (S1P).
© 2020 Ghazaly et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Morita Y., Perez G. I., Paris F., Miranda S. R., Ehleiter D., Haimovitz-Friedman A., Fuks Z., Xie Z., Reed J. C., Schuchman E. H., Kolesnick R. N., and Tilly J. L. (2000) Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 6, 1109–1114 10.1038/80442 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

