Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy
- PMID: 32161124
- PMCID: PMC7104341
- DOI: 10.1073/pnas.1921445117
Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy
Abstract
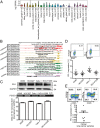
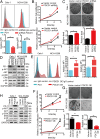
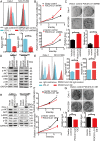
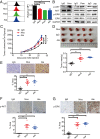
The programmed cell death 1 (PD-1) receptor on the surface of immune cells is an immune checkpoint molecule that mediates the immune escape of tumor cells. Consequently, antibodies targeting PD-1 have shown efficacy in enhancing the antitumor activity of T cells in some types of cancers. However, the potential effects of PD-1 on tumor cells remain largely unknown. Here, we show that PD-1 is expressed across a broad range of tumor cells. The silencing of PD-1 or its ligand, PD-1 ligand 1 (PD-L1), promotes cell proliferation and colony formation in vitro and tumor growth in vivo. Conversely, overexpression of PD-1 or PD-L1 inhibits tumor cell proliferation and colony formation. Moreover, blocking antibodies targeting PD-1 or PD-L1 promote tumor growth in cell cultures and xenografts. Mechanistically, the coordination of PD-1 and PD-L1 activates its major downstream signaling pathways including the AKT and ERK1/2 pathways, thus enhancing tumor cell growth. This study demonstrates that PD-1/PD-L1 is a potential tumor suppressor and potentially regulates the response to anti-PD-1/PD-L1 treatments, thus representing a potential biomarker for the optimal cancer immunotherapeutic treatment.
Keywords: biomarker; drug resistance; tumor cell-intrinsic PD-1; tumor cell-intrinsic PD-L1; tumor suppressor.
Conflict of interest statement
Competing interest statement: G.F.G. and C.X. are both members of the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS). G.F.G. and C.X. have not collaborated directly in this position.
Figures


References
-
- Bray F., et al. , Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018). - PubMed
-
- Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). - PubMed
-
- Vinay D. S., et al. , Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35 (suppl.), S185–S198 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

