Novel Insights into the Roles of Bcl-2 Homolog Nr-13 (vNr-13) Encoded by Herpesvirus of Turkeys in the Virus Replication Cycle, Mitochondrial Networks, and Apoptosis Inhibition
- PMID: 32161176
- PMCID: PMC7199394
- DOI: 10.1128/JVI.02049-19
Novel Insights into the Roles of Bcl-2 Homolog Nr-13 (vNr-13) Encoded by Herpesvirus of Turkeys in the Virus Replication Cycle, Mitochondrial Networks, and Apoptosis Inhibition
Abstract
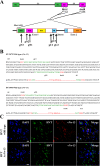
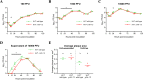
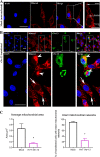
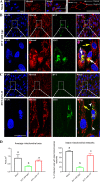
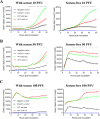
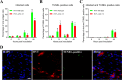
The Bcl-2 (B cell lymphoma 2)-related protein Nr-13 plays a major role in the regulation of cell death in developing avian B cells. With over 65% sequence similarity to the chicken Nr-13, herpesvirus of turkeys (HVT) vNr-13, encoded by the HVT079 and HVT096 genes, is the first known alphaherpesvirus-encoded Bcl-2 homolog. HVT-infected cells were reported to be relatively more resistant to serum starvation, suggested that vNr-13 could be involved in protecting the cells. Here, we describe CRISPR/Cas9-based editing of exon 1 of the HVT079 and HVT096 genes from the HVT genome to generate the mutant HVT-ΔvNr-13 to gain insights into its functional roles. Overall, wild-type HVT and HVT-ΔvNr-13 showed similar growth kinetics; however, at early time points, HVT-ΔvNr-13 showed 1.3- to 1.7-fold-lower growth of cell-associated virus and 3- to 6.2-fold-lower growth of cell-free virus. In transfected cells, HVT vNr-13 showed a mainly diffuse cytoplasmic distribution with faint nuclear staining. Further, vNr-13 localized to the mitochondria and endoplasmic reticulum (ER) and disrupted mitochondrial network morphology in the transfected cells. In the wild-type HVT-infected cells, vNr-13 expression appeared to be directly involved in the disruption of the mitochondrial network, as the mitochondrial network morphology was substantially restored in the HVT-ΔvNr-13-infected cells. IncuCyte S3 real-time apoptosis monitoring demonstrated that vNr-13 is unequivocally involved in the apoptosis inhibition, and it is associated with an increase of PFU, especially under serum-free conditions in the later stages of the viral replication cycle. Furthermore, HVT blocks apoptosis in infected cells but activates apoptosis in noninfected bystander cells.IMPORTANCE B cell lymphoma 2 (Bcl-2) family proteins play important roles in regulating apoptosis during homeostasis, tissue development, and infectious diseases. Several viruses encode homologs of cellular Bcl-2-proteins (vBcl-2) to inhibit apoptosis, which enable them to replicate and persist in the infected cells and to evade/modulate the immune response of the host. Herpesvirus of turkeys (HVT) is a nonpathogenic alphaherpesvirus of turkeys and chickens that is widely used as a live vaccine against Marek's disease and as recombinant vaccine viral vectors for protecting against multiple avian diseases. Identical copies of the HVT genes HVT079 and HVT096 encode the Bcl-2 homolog vNr-13. While previous studies have identified the potential ability of vNr-13 in inhibiting apoptosis induced by serum deprivation, there have been no detailed investigations on the functions of vNr-13. Using CRISPR/Cas9-based ablation of the vNr-13 gene, we demonstrated the roles of HVT vNr-13 in early stages of the viral replication cycle, mitochondrial morphology disruption, and apoptosis inhibition in later stages of viral replication.
Keywords: Bcl-2; Bcl-2 family; CRISPR; HVT-ΔvNr-13; apoptosis; vNr-13; wild-type HVT.
Copyright © 2020 Reddy et al.
Figures




References
-
- Witter RL, Nazerian K, Purchase HG, Burgoyne GH. 1970. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek’s disease virus. Am J Vet Res 31:525–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/I/00007032/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L014262/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K002465/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007030/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

