The Prognostic Role of Procalcitonin in Critically Ill Patients Admitted in a Medical Stepdown Unit: A Retrospective Cohort Study
- PMID: 32161314
- PMCID: PMC7066188
- DOI: 10.1038/s41598-020-61457-6
The Prognostic Role of Procalcitonin in Critically Ill Patients Admitted in a Medical Stepdown Unit: A Retrospective Cohort Study
Abstract
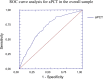
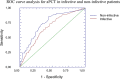
Procalcitonin (PCT) is a a marker of bacterial infection. Its prognostic role in the critically-ill patient, however, is still object of debate. Aim of this study was to evaluate the capacity of admission PCT (aPCT) in assessing the prognosis of the critically-ill patient regardless the presence of bacterial infection. A single-cohort, single-center retrospective study was performed evaluating critically-ill patients admitted to a stepdown care unit. Age, sex, Simplified Acute Physiology Score II (SAPS-II), shock, troponin-I, aPCT, serum creatinine, cultures and clinical endpoints (in-hospital mortality or Intensive Care Unit (ICU) transfer) were collected. Time free from adverse event (TF-AE) was defined as the time between hospitalization and occurrence of one of the clinical endpoints, and calculated with Kaplan-Meier curves. We engineered a new predictive model (POCS) adopting aPCT, age and shock.We enrolled 1063 subjects: 450 reached the composite outcome of death or ICU transfer. aPCT was significantly higher in this group, where it predicted TF-AE both in septic and non-septic patients. aPCT and POCS showed a good prognostic performance in the whole sample, both in septic and non-septic patients. aPCT showed a good prognostic accuracy, adding informations on the rapidity of clinical deterioration. POCS model reached a performance similar to SAPS-II.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

