Presynaptic calcium channels: specialized control of synaptic neurotransmitter release
- PMID: 32161339
- PMCID: PMC7873717
- DOI: 10.1038/s41583-020-0278-2
Presynaptic calcium channels: specialized control of synaptic neurotransmitter release
Abstract
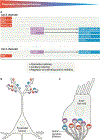
Chemical synapses are heterogeneous junctions formed between neurons that are specialized for the conversion of electrical impulses into the exocytotic release of neurotransmitters. Voltage-gated Ca2+ channels play a pivotal role in this process as they are the major conduits for the Ca2+ ions that trigger the fusion of neurotransmitter-containing vesicles with the presynaptic membrane. Alterations in the intrinsic function of these channels and their positioning within the active zone can profoundly alter the timing and strength of synaptic output. Advances in optical and electron microscopic imaging, structural biology and molecular techniques have facilitated recent breakthroughs in our understanding of the properties of voltage-gated Ca2+ channels that support their presynaptic functions. Here we examine the nature of these channels, how they are trafficked to and anchored within presynaptic boutons, and the mechanisms that allow them to function optimally in shaping the flow of information through neural circuits.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




References
-
- Katz B & Miledi R Ionic requirements of synaptic transmitter release. Nature 215, 651, (1967). - PubMed
-
- Wu J, Yan Z, Li Z, Qian X, Lu S, Dong M, Zhou Q & Yan N Structure of the voltage-gated calcium channel Cav1.1 at 3.6 A resolution. Nature 537, 191–196, (2016). - PubMed
-
- Zhao Y, Huang G, Wu Q, Wu K, Li R, Lei J, Pan X & Yan N Cryo-EM structures of apo and antagonist-bound human Cav3.1. Nature 576, 492–497, (2019). - PubMed
-
- Carbone E & Lux HD A low voltage-activated fully inactivating Ca channel in vertebrate sensory neurones. Nature 310, 501–502, (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

