Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics
- PMID: 32161484
- PMCID: PMC7065466
- DOI: 10.2147/CCID.S229054
Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics
Abstract
Compared to other domains, tissue engineering and esthetics have dramatically expanded in recent years, leading to both major biomedical advances and futuristic perspectives. The two share a common approach based on biomaterials, especially polymers. This paper illustrates this with the example of polycaprolactone (PCL), a polymer synthesized in the early 1930s, and one of its most recent applications, a PCL-based collagen stimulator, a filler used in esthetics. PCL is biocompatible and biodegradable. Its specific physicochemical and mechanical properties, viscoelasticity and ease of shaping led to the production of PCL-based products with various shapes and durations dependent on its biodegradation kinetics. PCL has been safely used in the biomedical field for more than 70 years, from sutures to tissue and organ replacement by 3D printing. The PCL-based collagen stimulator is composed of PCL microspheres suspended in a carboxymethyl-cellulose gel carrier providing immediate and sustained volumizing effects when injected; the morphology, the biocompatibility of the PCL microspheres embedded with the collagen fibers produced all contribute to the creation of a unique 3D scaffold for a sustained effect. Its safety has been investigated in clinical studies and vigilance surveys. Recently published experts' recommendations on injection modalities and techniques should help further optimize treatment outcome and safety. This paper also integrates reviews and recommendations on the prevention and management of adverse events related to dermal and subdermal fillers including the PCL-based collagen stimulator. In addition, in terms of efficacy and safety, this product benefits from its daily clinical use in esthetics worldwide and continuous extensive fundamental and clinical research, both on it and the PCL polymer. Forthcoming data from further investigations will reinforce knowledge of the product and procedures in the field.
Keywords: Ellansé®; collagen stimulators; dermal fillers; esthetics; polycaprolactone; safety.
© 2020 Christen and Vercesi.
Conflict of interest statement
The authors are consultants for Sinclair Pharma. Mrs Marie-Odile Christen reports personal fees from Sinclair Pharma, outside the submitted work. Dr Franco Vercesi is the International Key Opinion Leader for Sinclair Pharma. The authors report no other conflicts of interest in this work.
Figures

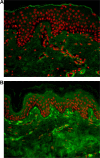


References
-
- American Society of Plastic Surgeons (ASPS). Plastic surgery statistics report; 2018. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-su.... Accessed August28, 2019.
-
- US Food & Drug Administration (FDA). Dermal fillers (soft tissue fillers). Available from: https://www.fda.gov/medical-devices/cosmetic-devices/dermal-fillers-soft.... Accessed August28, 2019.
-
- Agence Nationale de Sécurité du Médicament et des Produits de santé (ANSM). Topical report. Injectable products to fill wrinkles; July 2012. Available from: https://ansm.sante.fr/var/ansm_site/storage/original/application/0c98df1.... Accessed August28, 2019.
-
- Gritzalas K. Preliminary results in using a new dermal filler based on poly-caprolactone. Eur J Aesth Med Dermatol. 2011;1(1):22–26.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

