Role of the NLRP3 Inflammasome in Preeclampsia
- PMID: 32161574
- PMCID: PMC7053284
- DOI: 10.3389/fendo.2020.00080
Role of the NLRP3 Inflammasome in Preeclampsia
Abstract
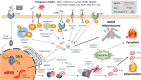
Reproduction involves tightly regulated series of events and the immune system is involved in an array of reproductive processes. Disruption of well-controlled immune functions leads to infertility, placental inflammation, and numerous pregnancy complications, including preeclampsia (PE). Inflammasomes are involved in the process of pathogen clearance and sterile inflammation. They are large multi-protein complexes that are located in the cytosol and play key roles in the production of the pivotal inflammatory cytokines, interleukin (IL)-1β and IL-18, and pyroptosis. The nucleotide-binding oligomerization domain, leucine-rich repeat-, and pyrin domain-containing 3 (NLRP3) inflammasome is a key mediator of sterile inflammation induced by various types of damage-associated molecular patterns (DAMPs). Recent evidence indicates that the NLRP3 inflammasome is involved in pregnancy dysfunction, including PE. Many DAMPs (uric acid, palmitic acid, high-mobility group box 1, advanced glycation end products, extracellular vesicles, cell-free DNA, and free fatty acids) are increased and associated with pregnancy complications, especially PE. This review focuses on the role of the NLRP3 inflammasome in the pathophysiology of PE.
Keywords: NLRP3 inflammasome; inflammation; interleukin-1β; preeclampsia; pregnancy.
Copyright © 2020 Shirasuna, Karasawa and Takahashi.
Figures
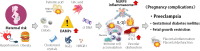
Similar articles
-
Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the NLRP3 inflammasomes.J Cell Physiol. 2019 May;234(5):5436-5450. doi: 10.1002/jcp.27475. Epub 2018 Oct 28. J Cell Physiol. 2019. PMID: 30370619 Review.
-
Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia.J Reprod Immunol. 2017 Sep;123:40-47. doi: 10.1016/j.jri.2017.09.002. Epub 2017 Sep 6. J Reprod Immunol. 2017. PMID: 28915449
-
β-hydroxybutyrate suppresses NLRP3 inflammasome-mediated placental inflammation and lipopolysaccharide-induced fetal absorption.J Reprod Immunol. 2021 Nov;148:103433. doi: 10.1016/j.jri.2021.103433. Epub 2021 Oct 5. J Reprod Immunol. 2021. PMID: 34628106
-
microRNA-520c-3p suppresses NLRP3 inflammasome activation and inflammatory cascade in preeclampsia by downregulating NLRP3.Inflamm Res. 2019 Aug;68(8):643-654. doi: 10.1007/s00011-019-01246-8. Epub 2019 May 30. Inflamm Res. 2019. PMID: 31143973
-
The role of mitochondria in NLRP3 inflammasome activation.Mol Immunol. 2018 Nov;103:115-124. doi: 10.1016/j.molimm.2018.09.010. Epub 2018 Sep 21. Mol Immunol. 2018. PMID: 30248487 Review.
Cited by
-
METTL3-mediated lncRNA HOXD-AS1 stability regulates inflammation, and the migration and invasion of trophoblast cells via the miR-135a/ β-TRCP axis.Noncoding RNA Res. 2023 Nov 11;9(1):12-23. doi: 10.1016/j.ncrna.2023.11.006. eCollection 2024 Mar. Noncoding RNA Res. 2023. PMID: 38075198 Free PMC article.
-
The role of pyroptosis in the occurrence and development of pregnancy-related diseases.Front Immunol. 2024 Sep 16;15:1400977. doi: 10.3389/fimmu.2024.1400977. eCollection 2024. Front Immunol. 2024. PMID: 39351226 Free PMC article. Review.
-
FABP4 facilitates inflammasome activation to induce the Treg/Th17 imbalance in preeclampsia via forming a positive feedback with IL-17A.Mol Ther Nucleic Acids. 2021 Apr 2;24:743-754. doi: 10.1016/j.omtn.2021.03.020. eCollection 2021 Jun 4. Mol Ther Nucleic Acids. 2021. Retraction in: Mol Ther Nucleic Acids. 2022 May 04;28:532. doi: 10.1016/j.omtn.2022.04.020. PMID: 33996256 Free PMC article. Retracted.
-
Cholesterol Crystals and NLRP3 Mediated Inflammation in the Uterine Wall Decidua in Normal and Preeclamptic Pregnancies.Front Immunol. 2020 Oct 8;11:564712. doi: 10.3389/fimmu.2020.564712. eCollection 2020. Front Immunol. 2020. PMID: 33117348 Free PMC article.
-
The NLRP3 inflammasome - interleukin 1β axis in uveal melanoma.FEBS Open Bio. 2023 Mar;13(3):545-555. doi: 10.1002/2211-5463.13566. Epub 2023 Feb 12. FEBS Open Bio. 2023. PMID: 36707938 Free PMC article.
References
-
- Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. (1952) 7:320–38.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

