Biochemical Characterization of Oyster and Clam Galectins: Selective Recognition of Carbohydrate Ligands on Host Hemocytes and Perkinsus Parasites
- PMID: 32161746
- PMCID: PMC7053492
- DOI: 10.3389/fchem.2020.00098
Biochemical Characterization of Oyster and Clam Galectins: Selective Recognition of Carbohydrate Ligands on Host Hemocytes and Perkinsus Parasites
Abstract
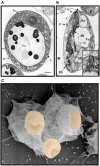
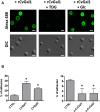
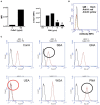
Both vertebrates and invertebrates display active innate immune mechanisms for defense against microbial infection, including diversified repertoires of soluble and cell-associated lectins that can effect recognition and binding to potential pathogens, and trigger downstream effector pathways that clear them from the host internal milieu. Galectins are widely distributed and highly conserved lectins that have key regulatory effects on both innate and adaptive immune responses. In addition, galectins can bind to exogenous ("non-self") carbohydrates on the surface of bacteria, enveloped viruses, parasites, and fungi, and function as recognition receptors and effector factors in innate immunity. Like most invertebrates, eastern oysters (Crassostrea virginica) and softshell clams (Mya arenaria) can effectively respond to most immune challenges through soluble and hemocyte-associated lectins. The protozoan parasite Perkinsus marinus, however, can infect eastern oysters and cause "Dermo" disease, which is highly detrimental to both natural and farmed oyster populations. The sympatric Perkinsus chesapeaki, initially isolated from infected M. arenaria clams, can also be present in oysters, and there is little evidence of pathogenicity in either clams or oysters. In this review, we discuss selected observations from our studies on the mechanisms of Perkinsus recognition that are mediated by galectin-carbohydrate interactions. We identified in the oyster two galectins that we designated CvGal1 and CvGal2, which strongly recognize P. marinus trophozoites. In the clam we also identified galectin sequences, and focused on one (that we named MaGal1) that also recognizes Perkinsus species. Here we describe the biochemical characterization of CvGal1, CvGal2, and MaGal1 with focus on the detailed study of the carbohydrate specificity, and the glycosylated moieties on the surfaces of the oyster hemocytes and the two Perkinsus species (P. marinus and P. chesapeaki). Our goal is to gain further understanding of the biochemical basis for the interactions that lead to recognition and opsonization of the Perkinsus trophozoites by the bivalve hemocytes. These basic studies on the biology of host-parasite interactions may contribute to the development of novel intervention strategies for parasitic diseases of biomedical interest.
Keywords: biochemical characterization; bivalve hemocyte; carbohydrate recognition; galectin; perkinsus parasites.
Copyright © 2020 Vasta, Feng, Tasumi, Abernathy, Bianchet, Wilson, Paschinger, Wang, Iqbal, Ghosh, Amin, Smith, Brown and Vista.
Figures

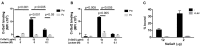





References
-
- Ahmed H., Bianchet M. A., Amzel L. M., Hirabayashi J., Kasai K., Giga-Hama Y., et al. (2002). Novel carbohydrate specificity of the 16-kDa galectin from Caenorhabditis elegans: binding to blood group precursor oligosaccharides (type 1, type 2, Tαβ, and Tβ) and gangliosides. Glycobiology 12, 451–461. 10.1093/glycob/cwf052 - DOI - PubMed
-
- Alavi M. R., Fernandez-Robledo J. A., Vasta G. R. (2009). Development of an in vitro assay to examine intracellular survival of Perkinsus marinus trophozoites upon phagocytosis by oyster (Crassostrea virginica and Crassostrea ariakensis) hemocytes. J. Parasitol. 95, 900–907. 10.1645/GE-1864.1 - DOI - PubMed
-
- Andrews J. D. (1996). History of Perkinsus marinus, a pathogen of oysters in Chesapeake Bay 1950-1984. J. Shellfish Res. 15, 13–16.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

