Hec1/Ndc80 Tail Domain Function at the Kinetochore-Microtubule Interface
- PMID: 32161753
- PMCID: PMC7054225
- DOI: 10.3389/fcell.2020.00043
Hec1/Ndc80 Tail Domain Function at the Kinetochore-Microtubule Interface
Abstract
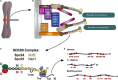
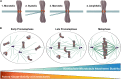
Successful mitotic cell division is critically dependent on the formation of correct attachments between chromosomes and spindle microtubules. Microtubule attachments are mediated by kinetochores, which are large proteinaceous structures assembled on centromeric chromatin of mitotic chromosomes. These attachments must be sufficiently stable to transduce force; however, the strength of these attachments are also tightly regulated to ensure timely, error-free progression through mitosis. The highly conserved, kinetochore-associated NDC80 complex is a core component of the kinetochore-microtubule attachment machinery in eukaryotic cells. A small, disordered region within the Hec1 subunit of the NDC80 complex - the N-terminal "tail" domain - has been actively investigated during the last decade due to its roles in generating and regulating kinetochore-microtubule attachments. In this review, we discuss the role of the NDC80 complex, and specifically the Hec1 tail domain, at the kinetochore-microtubule interface, and how recent studies provide a more unified view of Hec1 tail domain function.
Keywords: Hec1; NDC80; kinetochore; microtubule; mitosis.
Copyright © 2020 Wimbish and DeLuca.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

