Development of 3D neuromuscular bioactuators
- PMID: 32161837
- PMCID: PMC7064368
- DOI: 10.1063/1.5134477
Development of 3D neuromuscular bioactuators
Abstract
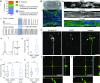
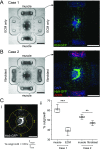
Neuronal control of skeletal muscle bioactuators represents a critical milestone toward the realization of future biohybrid machines that may generate complex motor patterns and autonomously navigate through their environment. Animals achieve these feats using neural networks that generate robust firing patterns and coordinate muscle activity through neuromuscular units. Here, we designed a versatile 3D neuron-muscle co-culture platform to serve as a test-bed for neuromuscular bioactuators. We used our platform in conjunction with microelectrode array electrophysiology to study the roles of synergistic interactions in the co-development of neural networks and muscle tissues. Our platform design enables co-culture of a neuronal cluster with up to four target muscle actuators, as well as quantification of muscle contraction forces. Using engineered muscle tissue targets, we first demonstrated the formation of functional neuromuscular bioactuators. We then investigated possible roles of long-range interactions in neuronal outgrowth patterns and observed preferential outgrowth toward muscles compared to the acellular matrix or fibroblasts, indicating muscle-specific chemotactic cues acting on motor neurons. Next, we showed that co-cultured muscle strips exhibited significantly higher spontaneous contractility as well as improved sarcomere assembly compared to muscles cultured alone. Finally, we performed microelectrode array measurements on neuronal cultures, which revealed that muscle-conditioned medium enhances overall neural firing rates and the emergence of synchronous bursting patterns. Overall, our study illustrates the significance of neuron-muscle cross talk for the in vitro development of neuromuscular bioactuators.
© Author(s).
Figures


References
LinkOut - more resources
Full Text Sources

