Vaccination With Moderate Coverage Eradicates Oncogenic Human Papillomaviruses If a Gender-Neutral Strategy Is Applied
- PMID: 32161969
- PMCID: PMC7430169
- DOI: 10.1093/infdis/jiaa099
Vaccination With Moderate Coverage Eradicates Oncogenic Human Papillomaviruses If a Gender-Neutral Strategy Is Applied
Abstract
Background: Human papillomavirus (HPV) vaccination of girls with very high (>90%) coverage has the potential to eradicate oncogenic HPVs, but such high coverage is hard to achieve. However, the herd effect (HE) depends both on the HPV type and the vaccination strategy.
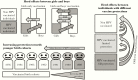
Methods: We randomized 33 Finnish communities into gender-neutral HPV16/18 vaccination, girls-only HPV16/18 vaccination, and hepatitis B virus vaccination arms. In 2007-2010, 11 662 of 20 513 of 40 852 of 39 420 resident boys/girls from 1992 to 1995 birth cohorts consented. In 2010-2014, cervicovaginal samples from vaccinated and unvaccinated girls at age 18.5 years were typed for HPV6/11/16/18/31/33/35/39/45/51/52/56/58/59/66/68. Vaccine efficacy for vaccinated girls, HE for unvaccinated girls, and the protective effectiveness (PE) for all girls were estimated. We extended the community-randomized trial results about vaccination strategy with mathematical modeling to assess HPV eradication.
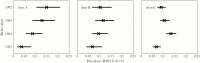
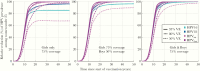
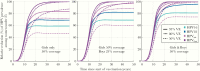
Results: The HE and PE estimates in the 1995 birth cohort for HPV18/31/33 were significant in the gender-neutral arm and 150% and 40% stronger than in the girls-only arm. Concordantly, HPV18/31/33 eradication was already predicted in adolescents/young adults in 20 years with 75% coverage of gender-neutral vaccination. With the 75% coverage, eventual HPV16 eradication was also predicted, but only with the gender-neutral strategy.
Conclusions: Gender-neutral vaccination is superior for eradication of oncogenic HPVs.
Trial registration: ClinicalTrials.gov NCT00534638.
Keywords: elimination; eradication; gender-neutral vaccination; herd effects; human papillomavirus.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Is It Now the Time to Plan for Global Gender-Neutral HPV Vaccination?J Infect Dis. 2020 Aug 17;222(6):888-889. doi: 10.1093/infdis/jiaa103. J Infect Dis. 2020. PMID: 32161971 No abstract available.
References
-
- European Medicines Agency. Human medicine European public assessment report (EPAR): Gardasil. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil. Accessed 14 October 2019.
-
- European Medicines Agency. Human medicine European public assessment report (EPAR): Cervarix. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/cervarix. Accessed 14 October 2019.
-
- Luostarinen T, Apter D, Dillner J, et al. . Vaccination protects against invasive HPV-associated cancer. Int J Cancer 2017; 42:2186–7. - PubMed
-
- Elfström KM, Dillner J, Arnheim-Dahlström L. Organization and quality of HPV vaccination programs in Europe. Vaccine 2015; 33:1673–81. - PubMed

