Clinico-pathologic features, treatment and outcomes of breast cancer during pregnancy or the post-partum period
- PMID: 32162192
- PMCID: PMC7398490
- DOI: 10.1007/s10549-020-05585-7
Clinico-pathologic features, treatment and outcomes of breast cancer during pregnancy or the post-partum period
Abstract
Purpose: Breast cancer during pregnancy (BC-P) or the first year post-partum (BC-PP) is rare and whether it differs from breast cancer (BC) in young women not associated with pregnancy is uncertain.
Methods: We queried our institutional database for BC-P and BC-PP cases and matched controls with BC not associated with pregnancy diagnosed between January 1, 1985 and December 31, 2013. We performed two parallel retrospective cohort studies evaluating clinico-pathologic features, treatment and outcomes for BC-P and BC-PP cases compared to their controls.
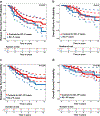
Results: In our population of 65 BC-P cases, 135 controls for BC-P cases, 75 BC-PP cases and 145 controls for BC-PP cases, high grade and estrogen receptor-negativity were more frequent in both case groups than their controls. Among those with stage I-III BC, patterns of local therapy were similar for both case groups and their controls, with the majority undergoing surgery and radiation. Over three-fourths of those with stage I-III BC received chemotherapy. BC-P cases tolerated chemotherapy well, with the majority receiving doxorubicin/cyclophosphamide every 3 weeks. On multivariate analyses of those with stage I-III BC, BC-P cases had non-significantly higher hazards of recurrence and death compared to their controls, while BC-PP cases had non-significantly lower hazards of recurrence and death compared to their controls.
Conclusion: BC-P and BC-PP were associated with adverse clinic-pathologic features in our population. However, we did not observe inferior outcomes for BC-P or BC-PP compared to controls, likely due to receipt of aggressive multi-modality therapy.
Keywords: Breast cancer; Post-partum; Pregnancy; Prognosis; Young women.
Conflict of interest statement
Figures
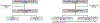
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

