Immunosuppressive agents for treating IgA nephropathy
- PMID: 32162319
- PMCID: PMC7066485
- DOI: 10.1002/14651858.CD003965.pub3
Immunosuppressive agents for treating IgA nephropathy
Abstract
Background: IgA nephropathy is the most common glomerulonephritis world-wide. IgA nephropathy causes end-stage kidney disease (ESKD) in 15% to 20% of affected patients within 10 years and in 30% to 40% of patients within 20 years from the onset of disease. This is an update of a Cochrane review first published in 2003 and updated in 2015.
Objectives: To determine the benefits and harms of immunosuppression strategies for the treatment of IgA nephropathy.
Search methods: We searched the Cochrane Kidney and Transplant Register of Studies up to 9 September 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs of treatment for IgA nephropathy in adults and children and that compared immunosuppressive agents with placebo, no treatment, or other immunosuppressive or non-immunosuppressive agents.
Data collection and analysis: Two authors independently assessed study risk of bias and extracted data. Estimates of treatment effect were summarised using random effects meta-analysis. Treatment effects were expressed as relative risk (RR) and 95% confidence intervals (95% CI) for dichotomous outcomes and mean difference (MD) and 95% CI for continuous outcomes. Risks of bias were assessed using the Cochrane tool. Evidence certainty was evaluated using GRADE methodology.
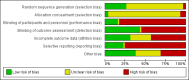
Main results: Fifty-eight studies involving 3933 randomised participants were included. Six studies involving children were eligible. Disease characteristics (kidney function and level of proteinuria) were heterogeneous across studies. Studies evaluating steroid therapy generally included patients with protein excretion of 1 g/day or more. Risk of bias within the included studies was generally high or unclear for many of the assessed methodological domains. In patients with IgA nephropathy and proteinuria > 1 g/day, steroid therapy given for generally two to four months with a tapering course probably prevents the progression to ESKD compared to placebo or standard care (8 studies; 741 participants: RR 0.39, 95% CI 0.23 to 0.65; moderate certainty evidence). Steroid therapy may induce complete remission (4 studies, 305 participants: RR 1.76, 95% CI 1.03 to 3.01; low certainty evidence), prevent doubling of serum creatinine (SCr) (7 studies, 404 participants: RR 0.43, 95% CI 0.29 to 0.65; low certainty evidence), and may lower urinary protein excretion (10 studies, 705 participants: MD -0.58 g/24 h, 95% CI -0.84 to -0.33;low certainty evidence). Steroid therapy had uncertain effects on glomerular filtration rate (GFR), death, infection and malignancy. The risk of adverse events with steroid therapy was uncertain due to heterogeneity in the type of steroid treatment used and the rarity of events. Cytotoxic agents (azathioprine (AZA) or cyclophosphamide (CPA) alone or with concomitant steroid therapy had uncertain effects on ESKD (7 studies, 463 participants: RR 0.63, 95% CI 0.33 to 1.20; low certainty evidence), complete remission (5 studies; 381 participants: RR 1.47, 95% CI 0.94 to 2.30; very low certainty evidence), GFR (any measure), and protein excretion. Doubling of serum creatinine was not reported. Mycophenolate mofetil (MMF) had uncertain effects on the progression to ESKD, complete remission, doubling of SCr, GFR, protein excretion, infection, and malignancy. Death was not reported. Calcineurin inhibitors compared with placebo or standard care had uncertain effects on complete remission, SCr, GFR, protein excretion, infection, and malignancy. ESKD and death were not reported. Mizoribine administered with renin-angiotensin system inhibitor treatment had uncertain effects on progression to ESKD, complete remission, GFR, protein excretion, infection, and malignancy. Death and SCr were not reported. Leflunomide followed by a tapering course with oral prednisone compared to prednisone had uncertain effects on the progression to ESKD, complete remission, doubling of SCr, GFR, protein excretion, and infection. Death and malignancy were not reported. Effects of other immunosuppressive regimens (including steroid plus non-immunosuppressive agents or mTOR inhibitors) were inconclusive primarily due to insufficient data from the individual studies in low or very low certainty evidence. The effects of treatments on death, malignancy, reduction in GFR at least of 25% and adverse events were very uncertain. Subgroup analyses to determine the impact of specific patient characteristics such as ethnicity or disease severity on treatment effectiveness were not possible.
Authors' conclusions: In moderate certainty evidence, corticosteroid therapy probably prevents decline in GFR or doubling of SCr in adults and children with IgA nephropathy and proteinuria. Evidence for treatment effects of immunosuppressive agents on death, infection, and malignancy is generally sparse or low-quality. Steroid therapy has uncertain adverse effects due to a paucity of studies. Available studies are few, small, have high risk of bias and generally do not systematically identify treatment-related harms. Subgroup analyses to identify specific patient characteristics that might predict better response to therapy were not possible due to a lack of studies. There is no evidence that other immunosuppressive agents including CPA, AZA, or MMF improve clinical outcomes in IgA nephropathy.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
No author has a vested interest in any of the products or procedures included in the analysis.
Figures
Update of
-
Immunosuppressive agents for treating IgA nephropathy.Cochrane Database Syst Rev. 2015 Aug 3;(8):CD003965. doi: 10.1002/14651858.CD003965.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2020 Mar 12;3:CD003965. doi: 10.1002/14651858.CD003965.pub3. PMID: 26235292 Updated.
References
References to studies included in this review
2nd NA IgAN 2004 {published data only}
-
- Hogg R, Bay C. Reduction of proteinuria observed in response to high dose ACE inhibition and omega‐3 fatty acids in pts with IgA nephropathy. Report from the second North American IgA nephropathy trial [abstract no: SA‐FC059]. Journal of the American Society of Nephrology 2007;18(Abstracts):48A. [CENTRAL: CN‐00740507]
-
- Hogg RJ. Preliminary report from the second North American IgA nephropathy (IgAN) trial [abstract no: F‐PO1098]. Journal of the American Society of Nephrology 2006;17(Abstracts):567A. [CENTRAL: CN‐00615827]
-
- Hogg RJ, Bay C. Dose‐dependency of the effect of omega‐3 fatty acids (O3FA) on proteinuria in patients with IgA nephropathy: report from the 2nd North American IgA nephropathy trial [abstract no: F‐PO860]. Journal of the American Society of Nephrology 2005;16:523A‐4A. [CENTRAL: CN‐00615826]
-
- Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, et al. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. American Journal of Kidney Diseases 2015;66(5):783‐91. [MEDLINE: ] - PubMed
-
- Hogg RJ, Fitzgibbons L, Atkins C, Nardelli N, Bay RC, North American IgA Nephropathy Study Group. Efficacy of omega‐3 fatty acids in children and adults with IgA nephropathy is dosage‐ and size‐dependent. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(6):1167‐72. [MEDLINE: ] - PubMed
Ballardie 2002 {published data only}
-
- Ballardie FW, Roberts IS. A controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy [abstract]. Nephrology Dialysis Transplantation 1996;11(8):1684. [CENTRAL: CN‐00261200] - PubMed
-
- Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. Journal of the American Society of Nephrology 2002;13(1):142‐8. [MEDLINE: ] - PubMed
BRIGHT‐SC 2016 {published data only}
-
- Barratt J, Hislop C, Pennington J, Gangal M, Martin R, Liew A. Effects of blisibimod, a selective inhibitor of b‐cell activating factor, in patients with IgA nephropathy [abstract no: FR‐PO1128]. Journal of the American Society of Nephrology 2016;27(Abstracts):4B. [CENTRAL: CN‐01658029]
Cao 2008 {published data only}
-
- Cao L, Ni Z, Qian J, Fang W, Lin A, Zhang W, et al. Leflunomide plus low dose prednisone reduced urinary VCAM‐1 level in progressive IgA nephropathy [abstract no: F‐PO1851]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):527A. [CENTRAL: CN‐00740501]
-
- Zhou M, Ni Z, Cao L, Qian J, Fang W, Shan M, et al. Leflunomide plus low dose prednisone reduced serum interleukin‐18 level in progressive IgA nephropathy [abstract no: TH‐PO515]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):232A.
CAST‐IgA 2015 {published data only}
-
- Kohagura K, Arima H, Miyasato H, Chang TH, Kobori H, Iseki K, et al. Effects of candesartan on clinical remission in IgA nephropathy treated with steroid pulse therapy and tonsilectomy (CAST IgA Study) ‐ a randomized control study [abstract no: TH‐PO661]. Journal of the American Society of Nephrology 2015;26(Abstracts):240A. [CENTRAL: CN‐01658023]
Chen 2002 {published data only}
-
- Chen X, Cai G, Zhang Y, Qiu Q, Cheng Q. Control study of effects of mycophenolate mofetil on IgA nephropathy [abstract no: A0311]. Journal of the American Society of Nephrology 2000;11(Sept):57A. [CENTRAL: CN‐00550413]
-
- Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, et al. A randomized control study of mycophenolate mofetil treatment in severe IgA nephropathy [abstract no: F‐FC065]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):14A. [CENTRAL: CN‐00444783]
-
- Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, et al. A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2002;82(12):796‐801. [MEDLINE: ] - PubMed
-
- Chen X, Wu J, Zhang Y, Liu S, Tang L. 72 weeks follow‐up study of effects of mycophenolate mofetil on IgA nephropathy [abstract no: A0353]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):66‐7A. [CENTRAL: CN‐00444784]
Cheung 2018 {published data only}
-
- Cheung CK, Szklarzewicz J, Rawal R, Barratt J. A phase II study evaluating the safety and efficacy of belimumab in patients with IgA nephropathy [abstract]. Kidney Diseases 2018;4(3):142‐3. [EMBASE: 624069857]
Cruzado 2011 {published data only}
-
- Cruzado JM, Poveda R, Ibernon M, Diaz M, Fulladosa X, Carrera M, et al. Low‐dose sirolimus combined with angiotensin‐converting enzyme inhibitor and statin stabilizes renal function and reduces glomerular proliferation in poor prognosis IgA nephropathy. Nephrology Dialysis Transplantation 2011;26(11):3596‐602. [MEDLINE: ] - PubMed
-
- Curzado JM, Poveda R, Fulladosa X, Torras J, Ibernon M, Diaz M, et al. Low dose of sirolimus for the treatment of poor‐prognosis IgA nephropathy: a prospective controlled trial [abstract no: F‐PO1964]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):555A. [CENTRAL: CN‐00757192]
Frisch 2005 {published data only}
-
- Frisch G, Lin J, Rosenstock J, Markowitz G, D'Agati V, Radhakrishnan J, et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double‐blind randomized controlled trial. Nephrology Dialysis Transplantation 2005;20(10):2139‐45. [MEDLINE: ] - PubMed
-
- Frisch G, Lin J, Rosenstock J, Markowitz G, D'Agati V, Radhakrishnan J, et al. Mycophenolate mofetil vs placebo in patients at high risk for progressive IgA nephropathy: a double blind RCT [abstract no: SU‐PO987]. Journal of the American Society of Nephrology 2003;14(Nov):753A. [CENTRAL: CN‐00644287]
Harmankaya 2002 {published data only}
-
- Harmankaya O, Ozturk Y, Basturk T, Obek A, Kilicarslan I. Efficacy of immunosuppressive therapy in IgA nephropathy presenting with isolated hematuria. International Urology & Nephrology 2002;33(1):167‐71. [MEDLINE: ] - PubMed
Hirai 2017 {published data only}
Horita 2007 {published data only}
-
- Horita Y, Tadokoro M, Taura K, Taguchi T, Kohno S. Effects of co‐administration of prednisolone plus losartan in moderate proteinuric IgA nephropathy [abstract no: MP099]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv332. [CENTRAL: CN‐00615873]
Hou 2017 {published data only}
-
- Hou JH, Le WB, Chen N, Wang WM, Liu ZS, Liu D, et al. Mycophenolate mofetil combined with prednisone versus full‐dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. American Journal of Kidney Diseases 2017;69(6):788‐95. [MEDLINE: ] - PubMed
Julian 1993 {published data only}
-
- Julian BA, Barker C. Alternate‐day prednisone therapy in IgA nephropathy. Preliminary analysis of a prospective, randomized, controlled trial. Contributions to Nephrology 1993;104:198‐206. [MEDLINE: ] - PubMed
-
- Julian BA, Barker CV, Woodford SY. Alternate‐day prednisone treatment of patients with IgA nephropathy [abstract no: 99P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):681. [CENTRAL: CN‐00484554]
Kanno 2003 {published data only}
-
- Kanno Y, Witt M, Okada H, Nemoto H, Sugahara S, Nakamoto H, et al. A comparison of corticosteroid and warfarin therapy in IgA nephropathy with crescent formation: preliminary trial. Clinical & Experimental Nephrology 2003;7(1):48‐51. [MEDLINE: ] - PubMed
Katafuchi 2003 {published data only}
-
- Katafuchi R, Ikeda K, Yanase T, Yanagida T, Yoshida T, Hirakata H, et al. A randomized prospective control study of low dose prednisolone therapy for IgA nephropathy: its usefulness and limitations [abstract]. Nephrology 1999;5(Suppl):A18. [CENTRAL: CN‐01657782]
-
- Katafuchi R, Kiyoshi I, Mizumasa T, Tanaka H, Ando T, Yanase T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low‐dose prednisolone therapy. American Journal of Kidney Diseases 2003;41(5):972‐83. [MEDLINE: ] - PubMed
-
- Katafuchi R, Yoshida T, Yanase T, Ikeda K, Fujimi S. Low dose steroid therapy in IgA nephropathy: a randomized prospective control study [abstract no: P1126]. Nephrology 1997;3(Suppl 1):S355. [CENTRAL: CN‐00461044]
Kawamura 2014 {published data only}
-
- Katafuchi R, Kawamura T, Hashiguchi A, Hisano S, Shimizu A, Miyazaki Y, et al. Pathological sub‐analysis of randomized controlled trial of tonsillectomy combined with steroid pulse therapy vs steroid pulse monotherapy in IGA nephropathy [abstract no: PS1‐096]. Nephrology 2014;19(Suppl 2):108. [EMBASE: 71617003] - PMC - PubMed
-
- Katafuchi R, Kawamura T, Joh K, Hashiguchi A, Hisano S, Shimizu A, et al. Pathological sub‐analysis of a multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy versus steroid pulse monotherapy in patients with immunoglobulin A nephropathy. Clinical & Experimental Nephrology 2016;20(2):244‐52. [MEDLINE: ] - PMC - PubMed
-
- Yoshimura M, Imasawa T, Nakayama M, Wada A, Katahuti R, Kawamura T. Tonsillectomy and steroid pulse therapy in IgA nephropathy: a randomized, controlled trial versus a multicenter prospective controlled study [abstract no: SU329]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐01912327]
Kim 2013b {published data only}
-
- Kim YC, Koo HS, Lee H, Chin HJ, Kim S. Double‐blind, randomized placebo‐controlled clinical trial for the effiicacy of tacrolimus in the patients with albuminuric, normotensive IgA nephropathy [abstract no: TH‐PO410]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):192A.
-
- Yu MY, Kim YC, Koo HS, Chin HJ. Short‐term anti‐proteinuric effect of tacrolimus is not related to preservation of the glomerular filtration rate in IgA nephropathy: a 5‐year follow‐up study.[Erratum in: PLoS One. 2018 Jan 29;13(1):e0192266; PMID: 29377948]. PLoS ONE [Electronic Resource] 2017;12(11):e0188375. [MEDLINE: ] - PMC - PubMed
Kobayashi 1996 {published data only}
-
- Kobayashi Y, Hiki Y, Kokubo T, Horii A, Tateno S. Steroid therapy during the early stage of progressive IgA nephropathy. A 10‐year follow‐up study. Nephron 1996;72(2):237‐42. [MEDLINE: ] - PubMed
Koike 2008 {published data only}
-
- Koike M, Takei T, Uchida K, Honda K, Moriyama T, Horita S, et al. Clinical assessment of low‐dose steroid therapy for patients with IgA nephropathy: a prospective study in a single center. Clinical & Experimental Nephrology 2008;12(4):250‐5. [MEDLINE: ] - PubMed
Koitabashi 1996 {published data only}
-
- Koitabashi Y, Yoshikawa N, Ito H. Randomized, prospective, multcenter trial for treatment of IgA nephropathy in children [abstract no: S‐5]. Pediatric Nephrology 1996;10(1):C4. [CENTRAL: CN‐01657792]
Lafayette 2017 {published data only}
-
- Lafayette RA, Canetta PA, Rovin BH, Appel GB, Hogan MC, Erickson SB, et al. A randomized trial of rituximab in advanced IgA nephropathy [abstract no: SA‐PO1098]. Journal of the American Society of Nephrology 2015;26(Abstracts):B5. [CENTRAL: CN‐01658018]
Lai 1986 {published data only}
-
- Lai KN, Lai FM, Ho CP, Chan KW. Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: a long‐term controlled trial. Clinical Nephrology 1986;26(4):174‐80. [MEDLINE: ] - PubMed
Lai 1987 {published data only}
-
- Lai KN, Lai FM. Short‐term controlled trial of cyclosporin A therapy in IgA nephropathy [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:73. [CENTRAL: CN‐00626035]
-
- Lai KN, Lai FM, Chui SH. Effect of cyclosporin A on cellular immunity in IgA nephropathy [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:388. [CENTRAL: CN‐00626034]
-
- Lai KN, Lai FM, Chui SH, Leung KN, Lam CW. Effect of ciclosporin on lymphocyte subpopulations and immunoglobulin production in IgA nephropathy. Nephron 1989;52(4):307‐12. [MEDLINE: ] - PubMed
-
- Lai KN, Lai FM, Vallance‐Owen J. Short‐term controlled trial of cyclosporin A in therapy in IgA nephropathy [abstract no: 12]. 23rd Annual Meeting Australasian Society of Nephrology; 1987 Apr 1‐3; Adelaide, SA. 1987:13.
Lee 2003 {published data only}
-
- Kim YK, Lee SH, Lee TW, Kim MJ, Yang MH, Ihm CG. Renoprotective effect of the combined use of steroid and angiotensin II receptor blocker in IgA Nephropathy. Korean Journal of Nephrology 2005;24(1):71‐9. [CENTRAL: CN‐01045483]
-
- Lee SH, Shim JJ, Lee SH, Lee TW, Kim MJ, Yang MH. Effect of combined treatment of steroid and angiotensin II receptor blocker (ARB) in proteinuric IgA nephropathy. Korean Journal of Nephrology 2003;22(5):539‐45. [CENTRAL: CN‐01045911]
Liu 2010a {published data only}
-
- Liu XW, Li DM, Xu GS, Sun SR. Comparison of the therapeutic effects of leflunomide and mycophenolate mofetil in the treatment of immunoglobulin A nephropathy manifesting with nephrotic syndrome. International Journal of Clinical Pharmacology & Therapeutics 2010;48(8):509‐13. [MEDLINE: ] - PubMed
Liu 2014 {published data only}
-
- Liu H, Ding X, Fang Y, Xu X, Zhang X, Zhong Y. Medium dose of cyclosporine combined with methylprednisolone in IgA nephropathy: prospective randomized controlled trial [abstract no: SA‐PO2300]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):636A.
-
- Liu H, Xu X, Fang Y, Ji J, Zhang X, Yuan M, et al. Comparison of glucocorticoids alone and combined with cyclosporine A in patients with IgA nephropathy: a prospective randomized controlled trial. Internal Medicine 2014;53(7):675‐81. [EMBASE: 2014229755] - PubMed
Locatelli 1999 {published data only}
-
- Vecchio L, Pozzi C, Andrulli S, Pani A, Scaini P, Fogazzi G, et al. Corticosteroids and azathioprine vs corticosteroids alone in IgA nephropathy: a randomised, controlled trial [abstract no: SA770]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐01912332]
-
- Locatelli F, Pozzi C, Vecchio L, Andrulli S, Pani A, Fogazzi G, et al. Combined treatment with steroids and azathioprine in IgA nephropathy: design of a prospective randomised multicentre trial. Journal of Nephrology 1999;12(5):308‐11. [MEDLINE: ] - PubMed
-
- Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, et al. IgA nephropathy with severe chronic renal failure: a randomized controlled trial of corticosteroids and azathioprine. Journal of Nephrology 2013;26(1):86‐93. [MEDLINE: ] - PubMed
Lou 2006 {published data only}
-
- Lou T, Wang C, Chen Z, Shi C, Tang H, Liu X, et al. Randomised controlled trial of leflunomide in the treatment of immunoglobulin A nephropathy. Nephrology 2006;11(2):113‐6. [MEDLINE: ] - PubMed
Lv 2009 {published data only}
-
- Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, et al. Combination therapy of prednisone and ACE inhibitor versus ACE‐inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. American Journal of Kidney Diseases 2009;53(1):26‐32. [MEDLINE: ] - PubMed
-
- Lv J, Zhang H, Chen Y, Li G, Wang H. Addition of steroids to ACE inhibitors is more preferred to patients with IgA nephropathy: a prospective randomized controlled trial [abstract no: SA‐FC105]. Journal of the American Society of Nephrology 2007;18(Abstracts):57A. [CENTRAL: CN‐00740506]
Maes 2004 {published data only}
-
- Maes B, Claes K, Evenepoel P, Kuypers D, Oyen R, Vanwalleghem J, et al. A prospective placebo‐controlled randomized study of mycophenolate mofetil treatment for IGA nephropathy: lack of clinical efficacy after three years [abstract no: T194]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):343‐4. [CENTRAL: CN‐00446533]
-
- Maes BD, Evenepoel P, Kuypers D, Messiaen T, Vanrenterghem Y. A prospective placebo‐controlled randomized single centre study of mycophenolate mofetil treatment for IGA nephropathy: lack of clinical efficacy after two years [abstract no: A0599]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):114A. [CENTRAL: CN‐00446534]
-
- Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, et al. Mycophenolate mofetil in IgA nephropathy: results of a 3‐year prospective placebo‐controlled randomized study. Kidney International 2004;65(5):1842‐9. [MEDLINE: ] - PubMed
Manno 2001 {published data only}
-
- Manno C, Gesualdo L, D'Altri C, Rossini M, Grandaliano G, Schena FP. Prospective randomized controlled multicenter trial on steroids plus ramipril in proteinuric IgA nephropathy. Journal of Nephrology 2001;14(4):248‐52. [MEDLINE: ] - PubMed
-
- Manno C, Torres DD, Pesce F, Rossini M, Schena FP. Long‐term prospective randomized controlled multicentre trial on steroids plus ramipril in proteinuric IgA nephropathy [abstract no: LB‐001]. American Society of Nephrology (ASN) Renal Week; 2008 Nov 4‐9; Philadelphia,PA. 2008. [CENTRAL: CN‐00740468]
-
- Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE‐inhibitors with long‐term follow‐up in proteinuric IgA nephropathy.[Erratum in: Nephrol Dial Transplant. 2010 Apr;25(4):1363‐4]. Nephrology Dialysis Transplantation 2009;24(12):3694‐701. [MEDLINE: ] - PubMed
-
- Torres D, Rossini M, Manno C, Gesualdo L, Grandaliano G, Schena FP, et al. Steroids plus ramipril versus ramipril alone in the treatment of IgA nephropathy: interim analysis of a prospective, controlled, randomized, multicenter trial [abstract no: MO27]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:222‐3. [CENTRAL: CN‐00636151]
Masutani 2016 {published data only}
-
- Masutani K, Tsuchimoto A, Yamada T, Hirakawa M, Mitsuiki K, Katafuchi R, et al. Comparison of steroid‐pulse therapy and combined with mizoribine in IgA nephropathy: a randomized controlled trial. Clinical & Experimental Nephrology 2016;20(6):896‐903. [MEDLINE: ] - PubMed
-
- Masutani K, Tsuchimoto A, Yamada T, Mitsuiki K, Katafuchi R, Hirakata HN, et al. No additional effect of oral immunosuppressive agents, mizoribine, with steroid pulse therapy in patients with IgA nephropathy: a prospective randomized controlled trial [abstract no: TH‐PO441]. Journal of the American Society of Nephrology 2014;25(Abstracts):207A. [CENTRAL: CN‐01658024]
Min 2017 {published data only}
NA IgAN 1995 {published data only}
-
- Hogg R, Fitzgibbons L, Lee JD, Julian BA, Holub BJ. Omega‐3 fatty acids (O3FA) for patients with IgA nephropathy (IgAN): efficacy is dose‐dependent. Report from the North American (NA) IgAN trial [abstract no: SA‐PO171]. Journal of the American Society of Nephrology 2004;15(Oct):337A. [CENTRAL: CN‐00676029]
-
- Hogg RJ. A randomized, placebo‐controlled, multicenter trial evaluating alternate‐day prednisone and fish oil supplements in young patients with immunoglobulin A nephropathy. Scientific Planning Committee of the IgA Nephropathy Study. American Journal of Kidney Diseases 1995;26(5):792‐6. [MEDLINE: ] - PubMed
-
- Hogg RJ, Fitzgibbons L, Atkins C, Nardelli N, Bay RC, North American IgA Nephropathy Study Group. Efficacy of omega‐3 fatty acids in children and adults with IgA nephropathy is dosage‐ and size‐dependent. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(6):1167‐72. [MEDLINE: ] - PubMed
-
- Hogg RJ, Lee J, Nardelli N, Julian BA, Cattran D, Waldo B, et al. Clinical trial to evaluate omega‐3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(3):467‐74. [MEDLINE: ] - PubMed
-
- Hogg RJ, Lee J, Nardelli NA, Cattran D, Hirschman G, Julian BA. Clinical trial of alternate‐day prednisone or daily omega‐3 fatty acids in patients with IgA nephropathy [abstract no: OFC10]. Pediatric Nephrology 2004;19(9):C64. [CENTRAL: CN‐00583302]
NEFIGAN 2017 {published data only}
-
- Bhachu JS, Scionti K, Muto M, Molyneux K, Barratt J. Targeted release‐budesonide (NEFECON) modifies circulating IGA‐IGG immune complex levels and levels of poorly O‐galactosylated IgA in IgAN [abstract]. Kidney Diseases 2018;4(3):121‐2. [EMBASE: 624087998]
-
- Fellstrom B, Barratt J, Cook H, Coppo R, Feehally J, Fijter J, et al. Proteinuria reduction in IgA nephropathy by nefecon, a targeted release formulation of budesonide ‐ results from the NEFIGAN trial [abstract no: TO013]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii82‐3. [EMBASE: 617291389]
-
- Fellstrom B, Barratt J, Floege J. Treatment of IgA nephropathy with nefecon, a targeted‐release formulation of budesonide‐extended posthoc results from the NEFIGAN trial [abstract]. Kidney Diseases 2018;4(3):140. [EMBASE: 624069810]
-
- Fellstrom B, Coppo R, Feehally J, Floege J, Jardine A, Locatelli F, et al. The NEFIGAN trial: a randomized, placebo‐controlled study to evaluate the efficacy and safety of NEFECON in IgA nephropathy patients at risk of developing ESRD: preliminary data from the run‐in phase [abstract no: TH‐PO442]. Journal of the American Society of Nephrology 2014;25(Abstracts):207A. [CENTRAL: CN‐01658027]
-
- Fellstrom B, Coppo R, Feehally J, Floege J, Fijter JW, Jardine AG, et al. The NEFIGAN trial: NEFECON, a novel targeted release formulation of budesonide, reduces proteinuria and stabilizes eGFR in IgA nephropathy patients at risk of ESRD [abstract no: HI‐OR04]. Journal of the American Society of Nephrology 2015;26(Abstracts):B1. [CENTRAL: CN‐01658026]
Ni 2005 {published data only}
-
- Ni Z, Qian J, Lu F, Jiang G, He L, Yao J, et al. Leflunomide plus low dose prednisone could reduce proteinuria and stabilize kidney function in progressive IgA nephropathy at 2 year follow‐up study [abstract no: F‐PO1967]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):555A. [CENTRAL: CN‐00740503]
-
- Ni Z, Qian JQ, Lu F, Yao J, Yuan WJ, Zhu H, et al. Leflunomide plus low dose prednisone therapy in progressive IgA nephropathy at 2 year follow‐up: a multi‐center, perspective, randomized control study [abstract no: SU‐PO1054]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):819A. [CENTRAL: CN‐00740500]
-
- Ni Z, Qian JQ, Lu F, Yao J, Yuan WJ, Zhu W, et al. Leflunomide treatment in progressive IgA nephropathy: interim analysis from a multi‐center, perspective, randomized control study [abstract no: SA‐PO1080]. Journal of the American Society of Nephrology 2006;17(Abstracts):800A. [CENTRAL: CN‐00740499]
-
- Ni Z, Qian JQ, Lu FM, Yao J, He L, Zhu H, et al. Leflunomide treatment in progressive IgA nephropathy: preliminary results from a multi‐center, perspective, randomized control study [abstract no: F‐PO859]. Journal of the American Society of Nephrology 2005;16:523A. [CENTRAL: CN‐00740496]
Nuzzi 2009 {published data only}
-
- Nuzzi F, D'Armiento M, Balletta MM, Malgieri G, Pecoraro C. Early corticosteroid treatment (CT) in children with IgA nephropathy (IGAn): a randomized and controlled trial [abstract]. Pediatric Nephrology 2010;25(9):1880. [EMBASE: 70438531]
-
- Nuzzi F, D'Armiento M, Malgieri G, Ferretti A, Marzano L, Pecoraro C. Early corticosteroid treatment in children with IGA nephropathy: a randomized and controlled trial [abstract no: OC005]. 27th Annual Scientific Meeting; Transplantation Society of Australia & New Zealand; 2009 June 17‐19; Canberra, Australia. 2009:28. [CENTRAL: CN‐00756254]
Pozzi 1999 {published data only}
-
- Vecchio L, Pozzi C, Fogazzi GB, Andrulli S, Pani A, Rustichelli R, et al. Renal histological picture and steroid treatment in IGA nephropathy [abstract no: SU‐PO984]. Journal of the American Society of Nephrology 2003;14(Nov):752A. [CENTRAL: CN‐00447275]
-
- Locatelli F, Pozzi C, Vecchio L, Bolasco PG, Fogazzi GB, Andrulli S, et al. Role of proteinuria reduction in the progression of IgA nephropathy. Renal Failure 2001;23(3‐4):495‐505. [MEDLINE: ] - PubMed
-
- Pozzi C. Randomized trial of steroids in IgA nephropathy with moderate proteinuria at 5 years of follow up [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A77. [CENTRAL: CN‐00250589]
-
- Pozzi C, Andrulli S, Vecchio L, Melis P, Fogazzi GB, Altieri P, et al. Corticosteroid effectiveness in IgA nephropathy: long‐term results of a randomized, controlled trial. Journal of the American Society of Nephrology 2004;15(1):157‐63. [MEDLINE: ] - PubMed
-
- Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 1999;353(9156):883‐7. [MEDLINE: ] - PubMed
Segarra 2006 {published data only}
-
- Segarra A, Vila J, Montoro B, Sunye P, Calero F, Orfila MA, et al. A multicenter randomized study to analyze the efficacy and safety of high‐dose immunoglobulin therapy associated with steroids vs steroid monotherapy in patients with IgA nephropathy [abstract no: MP085]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv327. [CENTRAL: CN‐00755295]
Shen 2013 {published data only}
-
- Shen P, Li Y, Wang Z, Wang W, Ren H, Zhang W, et al. A prospective randomized study on the efficacy of corticosteroid combined with cyclophosphamide or FK506 in primary IGA nephropathy with mild or moderate renal injury [abstract no: SP308]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i175. [EMBASE: 71075508]
Shi 2012a {published data only}
-
- Shi B, Ni Z, Cao L, Mou S, Zhang M, Wang Q, et al. Mannose‐binding lectin gene polymorphism may predict response to leflunomide in patients with progressive IgA nephropathy [abstract no: FR‐PO819]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):556A.
Shima 2018 {published data only}
-
- Shima Y, Nakanishi K, Kaku Y, Ishikura K, Hataya H, Matsuyama T, et al. Combination therapy with or without warfarin and dipyridamole for severe childhood IgA nephropathy: an RCT. Pediatric Nephrology 2018;33(11):2103‐12. [MEDLINE: ] - PubMed
Shoji 2000 {published data only}
-
- Shoji T, Nakanishi I, Saito N, Hayashi T, Togawa M, Okada N, et al. The corticosteroid treatment of diffuse mesangial proliferative IgA nephropathy: a one‐year prospective trial [abstract no: A0466]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):98A.
-
- Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, et al. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. American Journal of Kidney Diseases 2000;35(2):194‐201. [MEDLINE: ] - PubMed
Stangou 2011 {published data only}
-
- Stangou M, Ekonomidou D, Giamalis P, Liakou H, Tsiantoulas A, Pantzaki A, et al. Steroids and azathioprine in the treatment of IgA nephropathy. Clinical & Experimental Nephrology 2011;15(3):373‐80. [MEDLINE: ] - PubMed
STOP‐IgAN 2008 {published data only}
-
- Eitner F, Ackermann D, Hilgers RD, Floege J. Supportive versus immunosuppressive therapy of progressive IgA nephropathy (STOP) IgAN trial: rationale and study protocol. Journal of Nephrology 2008;21(3):284‐9. [MEDLINE: ] - PubMed
-
- Floege J, Rauen T, Eitner F, Fitzner C, Hilgers RD. Corticosteroid monotherapy versus combined immunosuppression in IgA nephropathy: insights from the STOP‐IgAN trial [abstract no: SA‐PO1097]. Journal of the American Society of Nephrology 2015;26(Abstracts):B4‐5. [CENTRAL: CN‐01658032]
-
- Floege J, Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, et al. Supportive versus immunosuppressive therapy for progressive IgA nephropathy (STOP‐IGAN): a randomized, controlled, open‐label multicenter trial [abstract]. 52nd Congress ERA‐EDTA; 2015 May 28‐31; London, UK. 2015. [CENTRAL: CN‐01658031]
-
- Floege J, Rauen T, Schindler J, Fitzner C, Eitner F, Groene HJ, et al. The MEST kidney biopsy score predicts renal outcome in STOP‐IgAN trial patients ‐ a post‐hoc study [abstract no: TH‐OR057]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):14A. [CENTRAL: CN‐01658030]
-
- Lennartz D, Rauen T, Fitzner C, Eitner F, Hilgers RD, Floege J. Dual blockade of the renin‐angiotensin system in patients with IgA nephropathy ‐ insights from the STOP‐IGAN Trial [abstract no: SUN‐021]. Kidney International Reports 2019;4(7 Suppl):S161. [EMBASE: 2002179482]
Takeda 1999 {published data only}
-
- Takeda T, Muso E, Maeda M, Ono T, Higashi Y, Takeshita K, et al. Two‐year randomized controlled trial of steroid therapy for adult patients with moderately active IgA nephropathy (IgAN) [abstract no: A0463]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):90A‐1A. [CENTRAL: CN‐00583221]
Tang 2005 {published data only}
-
- Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, et al. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney International 2005;68(2):802‐12. [MEDLINE: ] - PubMed
-
- Tang S, Leung JC, Tang AW, Ho YW, Chan LY, Chan TM, et al. A prospective, randomized, case‐controlled study on the efficacy of mycophenolate mofetil (MMF) for IgA nephropathy (IgAN) patients with persistent proteinuria despite angiotensin blockade [abstract no: SU‐PO986]. Journal of the American Society of Nephrology 2003;14(Nov):752A. [CENTRAL: CN‐00583218]
-
- Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN. Long‐term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney International 2010;77(6):543‐9. [MEDLINE: ] - PubMed
TESTING 2017 {published data only}
-
- Lv J, Zhang H, Perkovic V, TESTING Study Group. The therapeutic evaluation of steroids in IgA nephropathy global (TESTING) study [abstract no: LB01]. 53rd Congress ERA‐EDTA; 2016 May 21‐24; Vienna, Austria. 2016. [CENTRAL: CN‐01658019]
Walker 1990a {published data only}
-
- Walker RG, Yu SH, Owen JE, Kincaid‐Smith P. The treatment of mesangial IgA nephropathy with cyclophosphamide, dipyridamole and warfarin: a two‐year prospective trial. Clinical Nephrology 1990;34(3):103‐7. [MEDLINE: ] - PubMed
Welch 1992 {published data only}
-
- Welch TR, Fryer C, Shely E, Witte DP, Quinlan M. Double‐blind, controlled trial of short‐term prednisone therapy in immunoglobulin A glomerulonephritis. Journal of Pediatrics 1992;121(3):474‐7. [MEDLINE: ] - PubMed
Woo 1987 {published data only}
-
- Woo KT, Chiang GS, Lim CH. Follow‐up renal biopsies in IgA nephritic patients on triple therapy. Clinical Nephrology 1987;28(6):304‐5. [MEDLINE: ] - PubMed
-
- Woo KT, Chiang GS, Yap HK, Lim CH. Controlled therapeutic trial of IgA nephritis with follow‐up renal biopsies. Annals of the Academy of Medicine, Singapore 1988;17(2):226‐31. [MEDLINE: ] - PubMed
-
- Woo KT, Edmondson RP, Yap HK, Wu AY, Chiang GS, Lee EJ, et al. Effects of triple therapy on the progression of mesangial proliferative glomerulonephritis. Clinical Nephrology 1987;27(2):56‐64. [MEDLINE: ] - PubMed
-
- Woo KT, Lee GS, Lau YK, Chiang GS, Lim CH. Effects of triple therapy in IgA nephritis: a follow‐up study 5 years later. Clinical Nephrology 1991;36(2):60‐6. [MEDLINE: ] - PubMed
-
- Woo KT, Lee GS, Lau YK, Chiang GSC Lim CH. Anti platelet therapy in IgA nephritis [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:13. [CENTRAL: CN‐00448412]
Wu 2016 {published data only}
-
- Chen XM, Wu J, Duan S, Zheng Y. Efficacy and safety of telmisartan, clopidogrelin and leflunomide in patients with IgA nephropathy ‐ a multicentre, prospective, randomized, double‐blind, double‐dummy controlled clinical trial [abstract no: SA‐PO848]. Journal of the American Society of Nephrology 2013;24(Abstracts):821A. [CENTRAL: CN‐01658021]
-
- Wu J, Duan S, Li W, Wang Y, Liu W, Zhang J, et al. Efficacy and safety of telmisartan, clopidogrelin, and leflunomide in patients with IgA nephropathy ‐ a multicentre, prospective, randomized, double‐blind and‐dummy controlled clinical trial [abstract no: SP307]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i175. [EMBASE: 71075507]
Xie 2011 {published data only}
-
- Xie Y, Huang S, Wang L, Miao L, Zhang A, Li Y, et al. Efficacy and safety of mizoribine combined with losartan in the treatment of IgA nephropathy: a multicenter, randomized, controlled study. American Journal of the Medical Sciences 2011;341(5):367‐72. [MEDLINE: ] - PubMed
Yamauchi 2001 {published data only}
-
- Yamauchi A, Uzu T, Yamato M, Ko M, Takahara K. Effect of steroid therapy on the progression of IgA nephropathy [abstract no: A1329]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):259A. [CENTRAL: CN‐00448450]
Yoshikawa 1999 {published data only}
-
- Ito H, Yoshikawa N. Prospective multicenter controlled therapeutic trial in IgA nephropathy in Japanese children: a preliminary report [abstract no: S‐I‐2]. Pediatric Nephrology 1992;6(6):C208. [CENTRAL: CN‐01658022]
-
- Yoshikawa N, Ito H. Combined therapy with prednisolone, azathioprine, heparin‐warfarin, and dipyridamole for paediatric patients with severe IgA nephropathy‐‐is it relevant for adult patients?. Nephrology Dialysis Transplantation 1999;14(5):1097‐9. [MEDLINE: ] - PubMed
-
- Yoshikawa N, Ito H. Corticosteroids and immunosuppressive drugs [abstract]. Pediatric Nephrology 2001;16(8):C31. [CENTRAL: CN‐00448482]
-
- Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu, M, et al. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. Journal of the American Society of Nephrology 1999;10(1):101‐9. [MEDLINE: ] - PubMed
Yoshikawa 2006 {published data only}
-
- Yoshikawa N. Treatment of IGA nephropathy in children [abstract no: FCP04]. Pediatric Nephrology 2004;19(9):C57. [CENTRAL: CN‐00583685]
-
- Yoshikawa N, Honda M, Iijima K, Awazu M, Hattori S, Nakanishi K, et al. Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(3):511‐7. [MEDLINE: ] - PubMed
-
- Yoshikawa N, Ito H. Corticosteroids and immunosuppressive drugs [abstract]. Pediatric Nephrology 2001;16(8):C31. [CENTRAL: CN‐00448482]
-
- Yoshikawa N, Ito H. Prednisolone therapy versus combined therapy with prednisolone, azathioprine, warfarin and dipyridamole for newly diagnosed severe childhood IgA nephropathy: a controlled trial by the Japanese Pediatric IgA Nephropathy Treatment study group [abstract no: A0430]. Journal of the American Society of Nephrology 2000;11(Sept):79A. [CENTRAL: CN‐00550736] - PubMed
Zhang 2004 {published data only}
-
- Zhang XZ, He Q, Luo TC, Lin ST. Efficacy and safety of leflunomide in the treatment of IgA nephropathy: preliminary results from a randomized, corticosteroid controlled, multi‐center clinical trial [abstract no: SA‐PO168]. Journal of the American Society of Nephrology 2004;15(Oct):337A. [CENTRAL: CN‐00583916]
-
- Zhang XZ, He YC, Luo Q, Yang TC, Li XG, Lin SY. Efficacy and safety of leflunomide in the treatment of IgA nephropathy: a perspective, corticosteroid controlled, multi‐center clinical trial [abstract no: F‐PO1097]. Journal of the American Society of Nephrology 2006;17(Abstracts):567A. [CENTRAL: CN‐00644205]
References to studies excluded from this review
Chen 2009b {published data only}
-
- Chen Y, Qin Y. Clinical effects of triple therapy in treatment of IgA nephropathy patients with moderate proteinuria. Xian Dai Yi Yao Wei Sheng 2009;25:1645‐6. [CENTRAL: CN‐01912326]
Czock 2007 {published data only}
-
- Czock D, Rasche FM, Carius A, Glander P, Budde K, Bauer S, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid after enteric‐coated mycophenolate versus mycophenolate mofetil in patients with progressive IgA nephritis. Journal of Clinical Pharmacology 2007;47(7):850‐9. [MEDLINE: ] - PubMed
-
- Keller F, Mueller L, Rasche M, Carius A, Glander P, Bauer S, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid after enteric‐coated mycophenolate sodium and mycophenolate mofetil in patients with IgA nephritis and renal impairment [abstract no: F‐PO1099]. Journal of the American Society of Nephrology 2006;17(Abstracts):568A. [CENTRAL: CN‐00615866]
Dal Canton 2005 {published data only}
-
- Dal Canton A, Amore A, Barbano G, Coppo R, Emma F, Grandaliano G, et al. One‐year angiotensin‐converting enzyme inhibition plus mycophenolate mofetil immunosuppression in the course of early IgA nephropathy: a multicenter, randomised, controlled study. Journal of Nephrology 2005;18(2):136‐40. [MEDLINE: ] - PubMed
GloMY 2010 {published data only}
-
- Harper L. Randomised pilot trial of myfortic for the treatment of primary proteinuric glomerulonephritis (Short title: proteinuria in glomerulonephritis: Myfortic (GloMY)) Trial protocol – version 3.2. www.birmingham.ac.uk/Documents/college‐mds/trials/bctu/glomy/GloMY‐proto... (accessed prior to 13 January 2020).
Imai 2006 {published data only}
-
- Imai H, Hotta O, Yoshimura M, Konta T, Tsubakihara Y, Miyazaki M, et al. Deoxyspergualin, an immunosuppressant, in patients suffering from nephropathies with crescent formation: an open‐label trial to evaluate safety and efficacy. Clinical & Experimental Nephrology 2006;10(1):40‐54. [MEDLINE: ] - PubMed
Shen 2009 {published data only}
-
- Shen SJ, Hu ZX, Wang SM, Li QH. Effects of a combined regime of Tripterygium wilfordii glycosides and benazepril in treatment of IgA nephropathy. Zhong Guo Zhong Xi Yi jie He Shen Bing Za Zhi 2009;10:154‐5. [CENTRAL: CN‐01912324]
Sulimani 2001 {published data only}
-
- Sulimani FM, Alhomssi M, Mitwalli A, al Wakeel J, Alam A, Tarif N, et al. Difficult nephropathies: a multicenter randomized trial on the treatment [abstract]. Saudi Journal of Kidney Diseases & Transplantation 2001;12(2):229. [CENTRAL: CN‐00402775]
Yonemura 2000b {published data only}
-
- Yonemura K, Kimura M, Miyaji T, Hishida A. Short‐term effect of vitamin K administration on prednisolone‐induced loss of bone mineral density in patients with chronic glomerulonephritis. Calcified Tissue International 2000;66(2):123‐8. [MEDLINE: ] - PubMed
References to studies awaiting assessment
NCT00301600 {published data only}
-
- Li LS. Mycophenolate mofetil versus intravenous cyclophosphamide pulses in the treatment of crescentic IgA nephropathy. www.clinicaltrials.gov/ct2/show/NCT00301600 (first received 13 March 2006).
NCT02160132 {published data only}
-
- Deng Y. A controlled study of steroids therapy for patients of IgA nephropathy with active pathological changes. www.ClinicalTrials.gov/show/NCT02160132 2014; Vol. (first received 10 June 2014).
NCT02571842 {published data only}
-
- Chancharoenthana W. Rituximab in recurrent IgA nephropathy. www.ClinicalTrials.gov/show/NCT02571842 (first received 8 October 2015).
References to ongoing studies
AIGA 2016 {published data only}
-
- Kim C, Lee D, Lee S, Choi B, Han S, Lee S. Efficacy and safety of a combination of mycophenolate mofetil and corticosteroid in advanced IgA nephropathy (AIGA). www.clinicalTrials.gov/show/NCT02981212 (first received 5 December 2016).
ARTEMIS‐IgAN 2018 {published data only}
-
- NCT03608033. Study of the safety and efficacy of OMS721 in patients with immunoglobulin A (IgA) nephropathy. www.clinicaltrials.gov/show/nct03608033 (first received 31 July 2018).
ChiCTR1800014442 {published data only}
-
- Li Y, Fu RG. Prospective study of the efficacy and safety of improved Italy scheme therapy for IgA nephropathy. www.chictr.org.cn/showprojen.aspx?proj=24628 (first received 13 January 2018).
MAIN 2013 {published data only}
-
- Hou F. The effects of mycophenolate mofetil (MMF) on renal outcomes in advanced immunoglobulin A (IgA) nephropathy patients. www.ClinicalTrials.gov/show/NCT01854814 (first received 16 May 2013).
NCT00657059 {published data only}
-
- Yu X, Yang Q. Mycophenolate mofetil (MMF) in patients with IgA nephropathy (IgAN). www.clinicaltrials.gov/ct2/show/NCT00657059 (first received 14 April 2008).
NCT02808429 {published data only}
-
- NCT02808429. Efficacy and safety of atacicept in IgA nephropathy. www.ClinicalTrials.gov/show/NCT02808429 (first received 21 June 2016).
NCT03468972 {published data only}
-
- Han SH. Effect of immunosuppression in IgA nephropathy. www.clinicaltrials.gov/show/nct03468972 (first received 19 March 2018).
NEFIGARD 2018 {published data only}
-
- NCT03643965. Efficacy and safety of Nefecon in patients with primary IgA (immunoglobulin A) nephropathy (Nefigard). www.clinicaltrials.gov/ct2/show/NCT03643965 (first received 23 August 2018).
PIRAT 2015 {published data only}
-
- Berthoux F. Prevention in recipients with primary IgA Nephropathy of recurrence after kidney transplantation: ATG‐F versus basiliximab as induction immunosuppressive treatment (PIRAT). www.ClinicalTrials.gov/show/NCT02523768 (first received 14 August 2015).
SIGN 2014 {published data only}
-
- Tam WK, Tumlin J, Barratt J, Rovin HB, Roberts SD, Roufosse C, et al. Spleen tyrosine kinase (SYK) inhibition in IgA nephropathy: a global, phase ii, randomised placebo‐controlled trial of fostamatinib [abstract no: SUN‐036]. Kidney International Reports 2019;4(7 Suppl):S168. [EMBASE: 2002180224]
TIGER 2017 {published data only}
-
- Joly D, Alarmartine E. Treatment of IgA nephropathy according to renal lesions (TIGER). www.ClinicalTrials.gov/show/NCT03188887 (first received 16 June 2017).
TOPplus‐IgAN 2013 {published data only}
-
- Shi W. Extended follow‐up of treatment of prednisone plus cyclophosphamide in patients with advanced‐stage IgA nephropathy (e‐TOPplus). www.ClinicalTrials.gov/show/NCT03218852 (first received 17 July 2017}.
-
- Shi W. Treatment of prednisone plus cyclophosphamide in patients with advanced‐stage IgA nephropathy (TOPplus‐IgAN). www.ClinicalTrials.gov/show/NCT01758120 (first received 1 January 2013).
UMIN000032031 {published data only}
-
- Suzuki H. The steroid internal use method for patients with IgA nephropathy. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000036551 (first received 1 April 2018).
Additional references
Barbour 2019
Berger 1968
-
- Berger J, Hinglais N. Intercapillary deposits of IgA‐IgG [Les ddpots intercapillaires d'IgA‐IgG]. Journal d Urologie et de Nephrologie 1968;74(9):694‐5. [MEDLINE: ] - PubMed
Cattran 2009
-
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, at al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney International 2009;76(5):535‐45. [MEDLINE: ] - PubMed
Cheng 2015
-
- Cheng G, Liu D, Margetts P, Liu L, Zhao Z, Liu Z, et al. Valsartan combined with clopidogrel and/or leflunomide for the treatment of progressive immunoglobulin A nephropathy. Nephrology 2015;20(2):77‐84. [MEDLINE: ] - PubMed
Coppo 2018
-
- Coppo R. Treatment of IgA nephropathy: recent advances and prospects. Nephrologie et Therapeutique 2018;14 Suppl 1:S13–21. [MEDLINE: ] - PubMed
D'Amico 1987
-
- D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Quarterly Journal of Medicine 1987;64(245):709‐27. [MEDLINE: ] - PubMed
Egger 1997
Gale 2017
Gallo 1988
-
- Gallo GR, Katafuchi R, Neelakantappa K, Baldwin DS. Prognostic pathologic markers in IgA nephropathy. American Journal of Kidney Diseases 1988;12(5):362‐5. [MEDLINE: ] - PubMed
GRADE 2008
GRADE 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] - PubMed
Haas 2017
-
- Haas M, Verhave JC, Liu ZH, Alpers CE, Barratt J, Becker JU, et al. A multicenter study of the predictive value of crescents in IgA nephropathy.[Erratum in: J Am Soc Nephrol. 2017 May;28(5):1665; PMID: 28455358]. Journal of the American Society of Nephrology 2017;28(2):691‐701. [MEDLINE: ] - PMC - PubMed
Han 2010
-
- Han SH, Kang EW, Park JK, Kie JH, Han DS, Kang SW. Spontaneous remission of nephrotic syndrome in patients with IgA nephropathy. Nephrology Dialysis Transplantation 2010;26(5):1570–75. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hiki 2001
-
- Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, et al. Mass spectrometry proves under‐O‐glycosylation of glomerular IgA1 in IgA nephropathy. Kidney International 2001;59(3):1077‐85. [MEDLINE: ] - PubMed
Hirano 2019
Hogg 2015
-
- Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, et al. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. American Journal of Kidney Diseases 2015;66(5):783‐91. [MEDLINE: ] - PubMed
Hotta 2001
-
- Hotta O, Miyazaki M, Furuta T, Tomioka S, Chiba S, Horigome I, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. American Journal of Kidney Diseases 2001;38(4):736–43. [MEDLINE: ] - PubMed
Inagaki 2017
KDIGO 2012
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney International ‐ Supplement 2012;2(2):139‐274. [https://kdigo.org/wp‐content/uploads/2017/02/KDIGO‐2012‐GN‐Guideline‐Eng...
Kim 2016
-
- Kim J, Park JH, Kim DH, Kim HY, Kim SH, Park WD. A patient with IgA nephropathy: 5 years after complete remission of minimal change nephrotic syndrome. EWHA Medical Journal 2016;39(4):118‐21. [EMBASE: 613536188]
Kiryluk 2012
Lambers Heerspink 2015
-
- Lambers Heerspink HJ, Kropelin TF, Hoekman J, Zeeuw D, Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium. Drug‐induced reduction in albuminuria is associated with subsequent renoprotection: a meta‐analysis. American Journal of Kidney Diseases 2015;26(8):2055‐64. [MEDLINE: ] - PMC - PubMed
Liu 2019
Maillard 2015
Manno 2007
-
- Manno C, Strippoli GF, D'Altri C, Torres D, Rossini M, Schena FP. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. American Journal of Kidney Diseases 2007;49(6):763‐5. [MEDLINE: ] - PubMed
Mestecky 1993
-
- Mestecky J, Tomana M, Crowley‐Nowick PA, Moldoveanu Z, Julian BA, Jackson S. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contributions to Nephrology 1993;104:172‐82. [MEDLINE: ] - PubMed
Moriyama 2019
-
- Moriyama T. Clinical and histological features and therapeutic strategies for IgA nephropathy. Clinical & Experimental Nephrology 2019;23(9):1089‐99. [MEDLINE: ] - PubMed
NCT03841448
-
- A study of cemdisiran in adults with immunoglobulin A nephropathy (IgAN). www.clinicaltrials.gov/ct2/show/NCT03841448 (first posted 15 February 2019).
Neelakantappa 1988
-
- Neelakantappa K, Gallo GR, Baldwin DS. Proteinuria in IgA nephropathy. Kidney International 1988;33(3):716‐21. [MEDLINE: ] - PubMed
Nolin 1999
-
- Nolin L, Courteau M. Management of IgA nephropathy: evidence‐based recommendations. Kidney International ‐ Supplement 1999;70:S56‐62. [MEDLINE: ] - PubMed
Piccoli 2010
-
- Piccoli A, Codognotto M, Tabbi MG, Favaro E, Rossi B. Influence of tonsillectomy on the progression of mesangioproliferative glomerulonephritis. Nephrology Dialysis Transplantation 2010;25(8):2583–9. [MEDLINE: ] - PubMed
Pozzi 2010
Reich 2007
-
- Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. Journal of the American Society of Nephrology 2007;18(12):3177‐83. [MEDLINE: ] - PubMed
Reid 2011
Rekola 1991
-
- Rekola S, Bergstrand A, Bucht H. Deterioration of GFR in IgA nephropathy as measured by 51Cr‐EDTA clearance. Kidney International 1991;40(6):1050‐4. [MEDLINE: ] - PubMed
Rostoker 1995
-
- Rostoker G, Desvaux‐Belghiti D, Pilatte Y, Petit‐Phar M, Philippon C, Deforges L, et al. Immunomodulation with low‐dose immunoglobulins for moderate IgA nephropathy and Henoch‐Schonlein purpura. Preliminary results of a prospective uncontrolled trial. Nephron 1995;69(3):327‐34. [MEDLINE: ] - PubMed
Schena 2001
-
- Schena FP. Immunoglobulin A nephropathy with mild renal lesions: a call in the forest for physicians and nephrologists. American Journal of Medicine 2001;110(6):499‐500. [MEDLINE: ] - PubMed
Schena 2009
-
- Schena FP, Pesce F. Chapter 2: Epidemiology and ancestral difference. In: Kar Neng Lai editor(s). Recent advances in IgA nephropathy. Singapore: World Scientific, 2009:9‐19.
Schunemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Song 2017
Tesar 2015
Trimarchi 2017
-
- Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney International 2017;91(5):1014‐21. [MEDLINE: ] - PubMed
Wyatt 2001
-
- Wyatt RJ, Hogg RJ. Evidence‐based assessment of treatment options for children with IgA nephropathies. Pediatric Nephrology 2001;16(2):156‐67. [MEDLINE: ] - PubMed
Yang 2018
References to other published versions of this review
Samuels 2003a
Samuels 2003b
Samuels 2004
-
- Samuels JA, Strippoli GF, Craig JC, Schena FP, Molony DA. Immunosuppressive treatments for immunoglobulin A nephropathy: a meta‐analysis of randomized controlled trials. Nephrology 2004;9(4):177‐85. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous