Antidiabetic Drugs for the Risk of Alzheimer Disease in Patients With Type 2 DM Using FAERS
- PMID: 32162525
- PMCID: PMC11005324
- DOI: 10.1177/1533317519899546
Antidiabetic Drugs for the Risk of Alzheimer Disease in Patients With Type 2 DM Using FAERS
Abstract
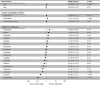
Alzheimer disease (AD) may develop after the onset of type 2 diabetes mellitus (T2DM), and the risk of AD may depend on the antidiabetic drug administered. We compared the risk of AD among 66 085 patients (≥ 65 years) with T2DM (1250 having concomitant AD) who had been administered antidiabetic drug monotherapy for T2DM who had voluntarily reported themselves in the Food and Drug Administration Adverse Event Reporting System. The risk of AD from the use of different antidiabetic drug monotherapies compared to that of metformin monotherapy was assessed by logistic regression. Rosiglitazone (adjusted reporting odds ratio [aROR] = 0.11; 95% confidence interval [CI]: 0.07-0.17; P < .001), exenatide (aROR = 0.22; 95% CI: 0.11-0.37; P < .001), liraglutide (aROR = 0.36; 95% CI: 0.19-0.62; P < .001), dulaglutide (aROR = 0.39; 95% CI: 0.17-0.77; P = .014), and sitagliptin (aROR = 0.75; 95% CI: 0.60-0.93; P = .011) were found to have a significantly lower associated risk of AD than that of metformin. Therefore, the administration of glucagon-like peptide 1 receptor agonists and rosiglitazone may reduce the risk of AD in patients with T2DM.
Keywords: Alzheimer disease; FDA adverse event reporting system; antidiabetics; dementia; type 2 diabetes mellitus.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. - PubMed
-
- Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Abeta amyloid peptides. Peptides. 2002;23(7):1285–1297. - PubMed
-
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. - PubMed
-
- Barage SH, Sonawane KD. Amyloid cascade hypothesis: pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides. 2015;52:1–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

