From Somatic Variants Toward Precision Oncology: An Investigation of Reporting Practice for Next-Generation Sequencing-Based Circulating Tumor DNA Analysis
- PMID: 32162803
- PMCID: PMC7066684
- DOI: 10.1634/theoncologist.2019-0239
From Somatic Variants Toward Precision Oncology: An Investigation of Reporting Practice for Next-Generation Sequencing-Based Circulating Tumor DNA Analysis
Abstract
Background: With the accelerated development of next-generation sequencing (NGS), identified variants, and targeted therapies, clinicians who confront the complicated and multifarious genetic information may not effectively incorporate NGS-based circulating tumor DNA (ctDNA) analysis into routine patient care. Consequently, standardized ctDNA testing reports are of vital importance. In an effort to guarantee high-quality reporting performance, we conducted an investigation of the current detection and reporting practices for NGS-based ctDNA analysis.
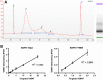
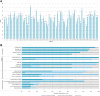
Materials and methods: A set of simulated ctDNA samples with known variants at known allelic frequencies and a corresponding case scenario were distributed to 66 genetic testing laboratories for ctDNA analysis. Written reports were collected to evaluate the detection accuracy, reporting integrity, and information sufficiency using 21 predefined criteria.
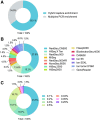
Results: Current reporting practices for NGS-based ctDNA analysis were found to be far from satisfactory, especially regarding testing interpretation and methodological details. Only 42.4% of laboratories reported the results in complete concordance with the expected results. Moreover, 74.2% of reports only listed aberrations with direct and well-known treatment consequences for the tumor type in question. Genetic aberrations for which experimental agents and/or drug access programs are available may thus be overlooked. Furthermore, methodological details for the interpretation of results were missing from the majority of reports (87.9%).
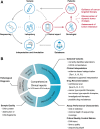
Conclusion: This proof-of-principle study suggests that the capacity for accurate identification of variants, rational interpretation of genotypes, comprehensive recommendation of potential medications, and detailed description of methodologies need to be further improved before ctDNA analysis can be formally implemented in the clinic.
Implications for practice: Accurate, comprehensive, and standardized clinical sequencing reports can help to translate complex genetic information into patient-centered clinical decisions, thereby shepherding precision oncology into daily practice. However, standards, guidelines, and quality requirements for clinical reports of next-generation sequencing (NGS)-based circulating tumor DNA (ctDNA) analysis are currently absent. By using a set of simulated clinical ctDNA samples and a corresponding case scenario, current practices were evaluated to identify deficiencies in clinical sequencing reports of ctDNA analysis. The recommendations provided here may serve as a roadmap for the improved implementation of NGS-based ctDNA analysis in the clinic.
Keywords: Circulating tumor DNA; Clinical reports; Next-generation sequencing; Quality control; Standardization.
© AlphaMed Press 2019.
Conflict of interest statement
Figures

Comment in
-
Completing the Translation.Oncologist. 2020 Mar;25(3):183-185. doi: 10.1634/theoncologist.2019-0650. Epub 2019 Oct 16. Oncologist. 2020. PMID: 32162829 Free PMC article.
References
-
- Oellerich M, Schütz E, Beck J et al. Using circulating cell‐free DNA to monitor personalized cancer therapy. Crit Rev Clin Lab Sci 2017;54:205–218. - PubMed
-
- Zugazagoitia J, Ramos I, Trigo JM et al. Clinical utility of plasma‐based digital next‐generation sequencing in patients with advance‐stage lung adenocarcinomas with insufficient tumor samples for tissue genotyping. Ann Oncol 2019;30:290–296. - PubMed
-
- Tomasetti M, Amati M, Neuzil J et al. Circulating epigenetic biomarkers in lung malignancies: From early diagnosis to therapy. Lung Cancer 2017;107:65–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

