Preliminary results of targeted prostate-specific membrane antigen imaging in evaluating the efficacy of a novel hormone agent in metastatic castration-resistant prostate cancer
- PMID: 32163676
- PMCID: PMC7221296
- DOI: 10.1002/cam4.2964
Preliminary results of targeted prostate-specific membrane antigen imaging in evaluating the efficacy of a novel hormone agent in metastatic castration-resistant prostate cancer
Abstract
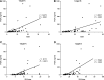
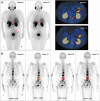
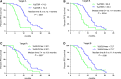
To investigate the feasibility and effectiveness of prostate-specific membrane antigen (PSMA) imaging to make response assessment regarding novel hormone treatment and to predict the outcomes for metastatic castration-resistant prostate cancer (mCRPC) patients. This retrospective study enrolled 68 mCRPC patients who had daily received a novel hormone agent named abiraterone. Tc-99m PSMA single-photon emission computed tomography (SPECT/CT) was performed at the baseline (SPECT/CT1) and after 3-6 months of treatment (SPECT/CT2). The treatment response was determined by visual analysis based on molecular imaging PSMA (miPSMA) scores framework and was compared with conventional biochemical analysis. We chose either the hottest lesion (target A) or five of the hottest lesions (target B) to calculate the tumor/background ratio (TBR) and the maximum standardized uptake value (SUVmax) and compared their performances in predicting progression-free survival (PFS). Changes in PSMA expression between SPECT/CT1 and SPECT/CT2 were well associated with the results of the visual analysis. The TBR and the SUVmax of both targets were significantly associated with the baseline serum PSA level (P < .0001). The biochemical and radiological responses were concordant in 56 of the 68 patients (P < .001). The median PFS of the nonresponse group patients was significantly shorter than that of the patients in the response group (6.8 vs 12.1 months, P = .012). For predicting PFS, most of the indexes tested were significant on SPECT/CT2, with %ΔTBR being the most significant prognostic factor. Our preliminary results suggest that molecular imaging-targeted PSMA is of great value for treatment response assessment and clinical outcome prediction in mCRPC patients with long-term abiraterone treatment.
Keywords: PSMA; SPECT/CT; abiraterone; mCRPC; response assessment.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures


References
-
- Sridhar SS, Freedland SJ, Gleave ME, et al. Castration‐resistant prostate cancer: from new pathophysiology to new treatment. Eur Urol. 2014;65(2):289‐299. - PubMed
-
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration‐resistant prostate cancer: final overall survival analysis of the COU‐AA‐301 randomised, double‐blind, placebo‐controlled phase 3 study. Lancet Oncol. 2012;13(10):983‐992. - PubMed
-
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy‐naive men with metastatic castration‐resistant prostate cancer (COU‐AA‐302): final overall survival analysis of a randomised, double‐blind, placebo‐controlled phase 3 study. Lancet Oncol. 2015;16(2):152‐160. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

