Ion Channels Controlling Circadian Rhythms in Suprachiasmatic Nucleus Excitability
- PMID: 32163720
- PMCID: PMC7717126
- DOI: 10.1152/physrev.00027.2019
Ion Channels Controlling Circadian Rhythms in Suprachiasmatic Nucleus Excitability
Abstract
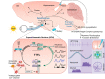
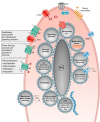
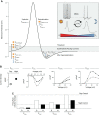
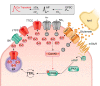
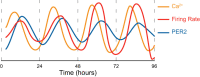
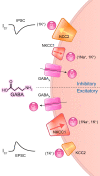
Animals synchronize to the environmental day-night cycle by means of an internal circadian clock in the brain. In mammals, this timekeeping mechanism is housed in the suprachiasmatic nucleus (SCN) of the hypothalamus and is entrained by light input from the retina. One output of the SCN is a neural code for circadian time, which arises from the collective activity of neurons within the SCN circuit and comprises two fundamental components: 1) periodic alterations in the spontaneous excitability of individual neurons that result in higher firing rates during the day and lower firing rates at night, and 2) synchronization of these cellular oscillations throughout the SCN. In this review, we summarize current evidence for the identity of ion channels in SCN neurons and the mechanisms by which they set the rhythmic parameters of the time code. During the day, voltage-dependent and independent Na+ and Ca2+ currents, as well as several K+ currents, contribute to increased membrane excitability and therefore higher firing frequency. At night, an increase in different K+ currents, including Ca2+-activated BK currents, contribute to membrane hyperpolarization and decreased firing. Layered on top of these intrinsically regulated changes in membrane excitability, more than a dozen neuromodulators influence action potential activity and rhythmicity in SCN neurons, facilitating both synchronization and plasticity of the neural code.
Keywords: action potential; circadian rhythm; excitability; ion channel; suprachiasmatic nucleus.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

