A Multiparameter Gas-Monitoring System Combining Functionalized and Non-Functionalized Microcantilevers
- PMID: 32164168
- PMCID: PMC7143659
- DOI: 10.3390/mi11030283
A Multiparameter Gas-Monitoring System Combining Functionalized and Non-Functionalized Microcantilevers
Abstract
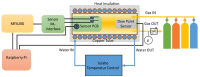
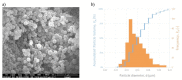
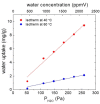
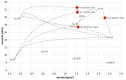
The aim of the study is to develop a compact, robust and maintenance free gas concentration and humidity monitoring system for industrial use in the field of inert process gases. Our multiparameter gas-monitoring system prototype allows the simultaneous measurement of the fluid physical properties (density, viscosity) and water vapor content (at ppm level) under varying process conditions. This approach is enabled by the combination of functionalized and non-functionalized resonating microcantilevers in a single sensing platform. Density and viscosity measuring performance is evaluated over a wide range of gases, temperatures and pressures with non-functionalized microcantilevers. For the humidity measurement, microporous Y-type zeolite and mesoporous silica MCM48 are evaluated as sensing materials. An easily scalable functionalization method to high-throughput production is herein adopted. Experimental results with functionalized microcantilevers exposed to water vapor (at ppm level) indicate that frequency changes cannot be attributed to a mass effect alone, but also stiffness effects dependent on adsorption of water and working temperature must be considered. To support this hypothesis, the mechanical response of such microcantilevers has been modelled considering both effects and the simulated results validated by comparison against experimental data.
Keywords: microcantilever; nanoporous functional coatings; ppm of water content; welding gas monitoring.
Conflict of interest statement
qisoteric isosteric isosteric heat of adsorption (J/mol)
Figures








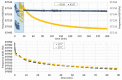
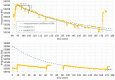
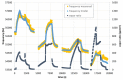
References
-
- ISO 14175:2008 . Welding Consumables—Gases and Gas Mixtures for Fusion Welding and Allied Processes. International Organization for Standardization ISO; Geneva, Switzerland: 2008.
-
- Huber C., Mehdaoui A., Pina M.P., Morales J.J. A Multiparameter Gas Monitoring System Combining Functionalized and Non-functionalized Microcantilevers; Proceedings of the 4th Conference on Microfluidic Handling Systems; Enschede, The Netherlands. 2–4 October 2019.
-
- Xu J., Bertke M., Wasisto H.S., Peiner E. Piezoresistive microcantilevers for humidity Sensing. J. Micromech. Microeng. 2019;29:053003. doi: 10.1088/1361-6439/ab0cf5. - DOI
-
- Ma R.-H., Lee C.-Y., Wang Y.-H., Chen H.-J. Microcantilever-based weather station for temperature, humidity and flow rate measurement. Microsyst Technol. 2008;14:971–977. doi: 10.1007/s00542-007-0458-2. - DOI
-
- Lee D., Shin N., Lee K.-H., Jeon S. Microcantilevers with nanowells as moisture sensors. Sens. Actuators B Chem. 2009;137:561–565. doi: 10.1016/j.snb.2009.01.031. - DOI
LinkOut - more resources
Full Text Sources

