Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis
- PMID: 32164386
- PMCID: PMC7140723
- DOI: 10.3390/cells9030680
Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis
Abstract
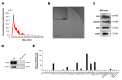
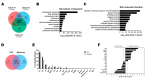
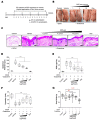
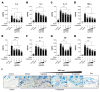
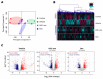
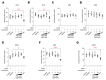
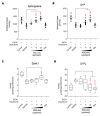
Atopic dermatitis (AD) is a multifactorial, heterogeneous disease associated with epidermal barrier disruption and intense systemic inflammation. Previously, we showed that exosomes derived from human adipose tissue-derived mesenchymal stem cells (ASC-exosomes) attenuate AD-like symptoms by reducing multiple inflammatory cytokine levels. Here, we investigated ASC-exosomes' effects on skin barrier restoration by analyzing protein and lipid contents. We found that subcutaneous injection of ASC-exosomes in an oxazolone-induced dermatitis model remarkably reduced trans-epidermal water loss, while enhancing stratum corneum (SC) hydration and markedly decreasing the levels of inflammatory cytokines such as IL-4, IL-5, IL-13, TNF-α, IFN-γ, IL-17, and TSLP, all in a dose-dependent manner. Interestingly, ASC-exosomes induced the production of ceramides and dihydroceramides. Electron microscopic analysis revealed enhanced epidermal lamellar bodies and formation of lamellar layer at the interface of the SC and stratum granulosum with ASC-exosomes treatment. Deep RNA sequencing analysis of skin lesions demonstrated that ASC-exosomes restores the expression of genes involved in skin barrier, lipid metabolism, cell cycle, and inflammatory response in the diseased area. Collectively, our results suggest that ASC-exosomes effectively restore epidermal barrier functions in AD by facilitating the de novo synthesis of ceramides, resulting in a promising cell-free therapeutic option for treating AD.
Keywords: ASC-exosomes; anti-inflammation; atopic dermatitis; ceramides; lamella body; restoration; skin barrier.
Conflict of interest statement
Y.W.Y. and B.S.C. are founders and stockholders of ExoCoBio Inc. D.H.H., J.O.K., H.-k.K., J.H.L., J.L., S.P., Y.W.Y., and B.S.C. are employees of ExoCoBio Inc. The other authors declare no conflict of interest.
Figures




References
-
- Deckers I.A., McLean S., Linssen S., Mommers M., van Schayck C.P., Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: A systematic review of epidemiological studies. PLoS ONE. 2012;7:e39803. doi: 10.1371/journal.pone.0039803. - DOI - PMC - PubMed
-
- Asher M.I., Montefort S., Björkstén B., Lai C.K., Strachan D.P., Weiland S.K., Williams H., ISAAC Phase Three Study Group Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. - DOI - PubMed
-
- Silverberg N.B., Silverberg J.I. Inside out or outside in: Does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

