How to Ease the Pain of Taking a Diagnostic Point of Care Test to the Market: A Framework for Evidence Development
- PMID: 32164393
- PMCID: PMC7142698
- DOI: 10.3390/mi11030291
How to Ease the Pain of Taking a Diagnostic Point of Care Test to the Market: A Framework for Evidence Development
Abstract
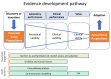
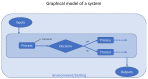
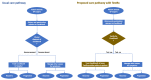
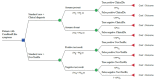
Bringing a diagnostic point of care test (POCT) to a healthcare market can be a painful experience as it requires the manufacturer to meet considerable technical, financial, managerial, and regulatory challenges. In this opinion article we propose a framework for developing the evidence needed to support product development, marketing, and adoption. We discuss each step in the evidence development pathway from the invention phase to the implementation of a new POCT in the healthcare system. We highlight the importance of articulating the value propositions and documenting the care pathway. We provide guidance on how to conduct care pathway analysis as little has been published on this. We summarize the clinical, economic and qualitative studies to be considered for developing evidence, and provide useful links to relevant software, on-line applications, websites, and give practical advice. We also provide advice on patient and public involvement and engagement (PPIE), and on product management. Our aim is to help device manufacturers to understand the concepts and terminology used in evaluation of in vitro diagnostics (IVDs) so that they can communicate effectively with evaluation methodologists, statisticians, and health economists. Manufacturers of medical tests and devices can use the proposed framework to plan their evidence development strategy in alignment with device development, applications for regulatory approval, and publication.
Keywords: adoption; care pathway analysis; diagnostic; evidence generation; implementation; medical device; point of care; preparation for marketing; value proposition.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the conception of this study or in the writing of the manuscript.
Figures
References
-
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. [(accessed on 2 March 2020)]; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0746.
-
- NICE Evidence Standards Framework for Digital Health Technologies. [(accessed on 2 March 2020)]; Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standar....
-
- DHSC Code of Conduct for Data-Driven Health and Care Technology. [(accessed on 2 March 2020)]; Available online: https://www.gov.uk/government/publications/code-of-conduct-for-data-driv....
-
- NICE Diagnostics Assessment Programme Manual. [(accessed on 2 March 2020)]; Available online: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NIC....
Grants and funding
LinkOut - more resources
Full Text Sources

