Senescence in Post-Mitotic Cells: A Driver of Aging?
- PMID: 32164429
- PMCID: PMC7821432
- DOI: 10.1089/ars.2020.8048
Senescence in Post-Mitotic Cells: A Driver of Aging?
Abstract
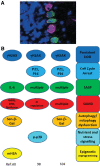
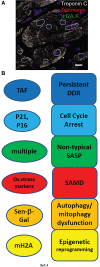
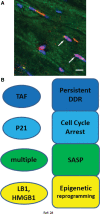
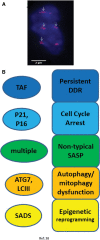
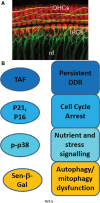
Significance: Cell senescence was originally defined by an acute loss of replicative capacity and thus believed to be restricted to proliferation-competent cells. More recently, senescence has been recognized as a cellular stress and damage response encompassing multiple pathways or senescence domains, namely DNA damage response, cell cycle arrest, senescence-associated secretory phenotype, senescence-associated mitochondrial dysfunction, autophagy/mitophagy dysfunction, nutrient and stress signaling, and epigenetic reprogramming. Each of these domains is activated during senescence, and all appear to interact with each other. Cell senescence has been identified as an important driver of mammalian aging. Recent Advances: Activation of all these senescence domains has now also been observed in a wide range of post-mitotic cells, suggesting that senescence as a stress response can occur in nondividing cells temporally uncoupled from cell cycle arrest. Here, we review recent evidence for post-mitotic cell senescence and speculate about its possible relevance for mammalian aging. Critical Issues: Although a majority of senescence domains has been found to be activated in a range of post-mitotic cells during aging, independent confirmation of these results is still lacking for most of them. Future Directions: To define whether post-mitotic senescence plays a significant role as a driver of aging phenotypes in tissues such as brain, muscle, heart, and others. Antioxid. Redox Signal. 34, 308-323.
Keywords: aging; post-mitotic; senescence.
Figures

References
-
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, and Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990, 2013 - PMC - PubMed
-
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d'Adda di Fagagna F, Bernard D, Hernando E, and Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018, 2008 - PubMed
-
- Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13: 40–47, 2003 - PubMed
-
- Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, Proctor C, Correia-Melo C, Victorelli S, Fielder E, Berlinguer-Palmini R, Owens A, Greaves LC, Kolsky KL, Parini A, Douin-Echinard V, LeBrasseur NK, Arthur HM, Tual-Chalot S, Schafer MJ, Roos CM, Miller JD, Robertson N, Mann J, Adams PD, Tchkonia T, Kirkland JL, Mialet-Perez J, Richardson GD, and Passos JF. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J 38 [Epub ahead of print]; DOI: 10.15252/embj/2018100492, 2019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
