Temporal decline in diarrhea episodes and mortality in Kiribati children two years following rotavirus vaccine introduction, despite high malnutrition rates: a retrospective review
- PMID: 32164562
- PMCID: PMC7069014
- DOI: 10.1186/s12879-020-4874-6
Temporal decline in diarrhea episodes and mortality in Kiribati children two years following rotavirus vaccine introduction, despite high malnutrition rates: a retrospective review
Abstract
Background: Kiribati introduced rotavirus vaccine in 2015. To estimate the impact of rotavirus vaccine on acute gastroenteritis (AGE) and severe acute malnutrition (SAM) among children under 5 in Kiribati, a retrospective review of inpatient and outpatient AGE and hospitalized SAM was undertaken.
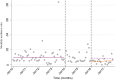
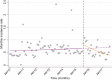
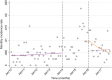
Methods: Inpatient data for admissions and hospital deaths due to AGE, SAM and all-causes were collected for children under 5 from all hospitals on the main island, Tarawa, from January 2010-December 2013 (pre-rotavirus vaccine) and January 2016-September 2017 (post-rotavirus vaccine). National outpatient diarrhea data were collected from January 2010 to August 2017 for under 5. An interrupted time-series analysis was undertaken to estimate the effect of rotavirus vaccine on the rates of inpatient and outpatient AGE, inpatient SAM; and inpatient case fatality rates for AGE and SAM, were calculated pre- and post-rotavirus vaccine introduction.
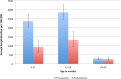
Results: The incidence rate of AGE admissions from Tarawa and national AGE outpatient presentations significantly declined by 37 and 44%, respectively, 2 years following rotavirus vaccine introduction. There was a significant decline in the percentage of AGE contributing to all-cause under 5 admissions (12·8% vs. 7·2%, p < 0·001) and all-cause under-five mortality (15·9% vs. 5·7%, p = 0·006) pre- and post-rotavirus vaccine introduction. The estimated incidence rate of inpatient SAM decreased by 24% in under 5 s, 2 years following rotavirus vaccine introduction.
Conclusions: AGE morbidity and mortality and hospitalized SAM rates have declined following rotavirus vaccine introduction in Kiribati children.
Keywords: Diarrheal disease; Hospital; Kiribati; Rotavirus vaccine; Severe acute malnutrition.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, Kang G, Kirkpatrick BD, Kirkwood CD, Mwenda JM, Parashar UD, Pertri WA, Riddle MS, Steele D, Thompson RL, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SI, Reiner RC. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):505–511. doi: 10.1001/jamapediatrics.2018.1960. - DOI - PMC - PubMed
-
- Heymann DL. Control of communicable disease manual. 20. United States of America: American Public Health Association; 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

