Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma - a case series
- PMID: 32164575
- PMCID: PMC7066743
- DOI: 10.1186/s12885-020-6589-x
Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma - a case series
Erratum in
-
Correction to: Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma - a case series.BMC Cancer. 2020 Apr 14;20(1):301. doi: 10.1186/s12885-020-06783-8. BMC Cancer. 2020. PMID: 32290821 Free PMC article.
Abstract
Background: The expression of vascular endothelial growth factor (VEGF)-A/ VAGF receptors (VEGFRs) signaling plays a pivotal role in the tumor angiogenesis and the development of the immunosuppressive tumor microenvironment in glioblastomas. We have previously conducted exploratory clinical studies investigating VEGFRs peptide vaccination with and without multiple glioma oncoantigens in patients with recurrent high-grade gliomas. Recently, an exploratory clinical investigation of VEGFRs peptide vaccination was conducted in patients with progressive neurofibromatosis type 2. Those studies suggested that cytotoxic T lymphocytes (CTLs) induced by the vaccination can directly kill a wide variety of cells associated with tumor growth, including tumor vessels, tumor cells, and immunosuppressive cells expressing VEGFR1 and/or 2. In the present study, synergistic activity of the combination of VEGFRs peptide vaccination with chemotherapy was evaluated.
Methods: We performed the first clinical trial to assess VEGFR1 and 2 vaccination along with temozolomide (TMZ) -based chemoradiotherapy for the patients with primary glioblastomas. Furthermore, histopathological changes after the vaccination were evaluated using paired pre- and post- vaccination specimens.
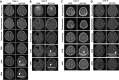
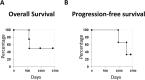
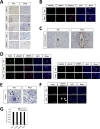
Results: The disappearance of radiographically enhanced lesion was observed in 2 patients after the vaccination, including one in which the methylation of the O6-methylguanine-DNA methyltransferase (MGMT) promoter was not observed. The histopathological findings of pre- and post-vaccination specimens demonstrated that tumor vessels showed negative or slight VEGFRs expressions after the vaccination and most endothelial cells were covered with PDGFR-β-positive pericytes. Notably, CTLs induced by VEGFRs peptide vaccination attacked not only tumor vessels but also tumor cells and regulatory T cells expressing VEGFRs even in recurrent tumors.
Conclusions: VEGFR1 and 2 vaccination may have a preliminary synergistic effect when administered with TMZ. The limitation of the present study was the paucity of the number of the samples. Further studies involving more patients are warranted to confirm the findings of this study.
Trial registration: This study was registered as UMIN000013381 (University Hospital Medical Information Network-Clinical Trial Registry: UMIN-CTR) on 5 March, 2014 and with the Japan Registry of Clinical Trials (jRCT) as jRCTs031180170 on 1 March, 2019.
Keywords: Bevacizumab; Glioblastoma; Peptide vaccine; VEGFR.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
A VEGF receptor vaccine demonstrates preliminary efficacy in neurofibromatosis type 2.Nat Commun. 2019 Dec 17;10(1):5758. doi: 10.1038/s41467-019-13640-1. Nat Commun. 2019. PMID: 31848332 Free PMC article. Clinical Trial.
-
Long-term therapy with temozolomide is a feasible option for newly diagnosed glioblastoma: a single-institution experience with as many as 101 temozolomide cycles.Neurosurg Focus. 2014 Dec;37(6):E4. doi: 10.3171/2014.9.FOCUS14502. Neurosurg Focus. 2014. PMID: 25434389
-
Aspirin Affects Tumor Angiogenesis and Sensitizes Human Glioblastoma Endothelial Cells to Temozolomide, Bevacizumab, and Sunitinib, Impairing Vascular Endothelial Growth Factor-Related Signaling.World Neurosurg. 2018 Dec;120:e380-e391. doi: 10.1016/j.wneu.2018.08.080. Epub 2018 Aug 23. World Neurosurg. 2018. PMID: 30144594
-
Anti-glioma therapy with temozolomide and status of the DNA-repair gene MGMT.Anticancer Res. 2009 Nov;29(11):4845-54. Anticancer Res. 2009. PMID: 20032445 Review.
-
Drug Insight: temozolomide as a treatment for malignant glioma--impact of a recent trial.Nat Clin Pract Neurol. 2005 Dec;1(2):88-95. doi: 10.1038/ncpneuro0045. Nat Clin Pract Neurol. 2005. PMID: 16932504 Review.
Cited by
-
Advances in Polymeric Micelles: Responsive and Targeting Approaches for Cancer Immunotherapy in the Tumor Microenvironment.Pharmaceutics. 2023 Nov 13;15(11):2622. doi: 10.3390/pharmaceutics15112622. Pharmaceutics. 2023. PMID: 38004600 Free PMC article. Review.
-
Correction to: Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma - a case series.BMC Cancer. 2020 Apr 14;20(1):301. doi: 10.1186/s12885-020-06783-8. BMC Cancer. 2020. PMID: 32290821 Free PMC article.
-
Small Molecule Tyrosine Kinase Inhibitors (TKIs) for Glioblastoma Treatment.Int J Mol Sci. 2024 Jan 23;25(3):1398. doi: 10.3390/ijms25031398. Int J Mol Sci. 2024. PMID: 38338677 Free PMC article. Review.
-
Overcoming temozolomide resistance in glioma: recent advances and mechanistic insights.Acta Neuropathol Commun. 2025 Jun 5;13(1):126. doi: 10.1186/s40478-025-02046-4. Acta Neuropathol Commun. 2025. PMID: 40468460 Free PMC article. Review.
-
Antiangiogenic Targets for Glioblastoma Therapy from a Pre-Clinical Approach, Using Nanoformulations.Int J Mol Sci. 2020 Jun 24;21(12):4490. doi: 10.3390/ijms21124490. Int J Mol Sci. 2020. PMID: 32599834 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

