Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells
- PMID: 32164731
- PMCID: PMC7068958
- DOI: 10.1186/s12951-020-00604-7
Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells
Abstract
Background: Metastasis causes the most breast cancer-related deaths in women. Here, we investigated the antitumor effect of solid lipid nanoparticles (SLN-DTX) when used in the treatment of metastatic breast tumors using 4T1-bearing BALB/c mice.
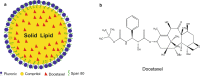
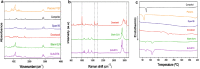
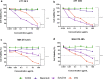
Results: Solid lipid nanoparticles (SLNs) were produced using the high-energy method. Compritol 888 ATO was selected as the lipid matrix, and Pluronic F127 and Span 80 as the surfactants to stabilize nanoparticle dispersion. The particles had high stability for at least 120 days. The SLNs' dispersion size was 128 nm, their polydispersity index (PDI) was 0.2, and they showed a negative zeta potential. SLNs had high docetaxel (DTX) entrapment efficiency (86%), 2% of drug loading and showed a controlled drug-release profile. The half-maximal inhibitory concentration (IC50) of SLN-DTX against 4T1 cells was more than 100 times lower than that of free DTX after 24 h treatment. In the cellular uptake test, SLN-DTX was taken into the cells significantly more than free DTX. The accumulation in the G2-M phase was significantly higher in cells treated with SLN-DTX (73.7%) than in cells treated with free DTX (23.0%), which induced subsequent apoptosis. TEM analysis revealed that SLN-DTX internalization is mediated by endocytosis, and fluorescence microscopy showed DTX induced microtubule damage. In vivo studies showed that SLN-DTX compared to free docetaxel exhibited higher antitumor efficacy by reducing tumor volume (p < 0.0001) and also prevented spontaneous lung metastasis in 4T1 tumor-bearing mice. Histological studies of lungs confirmed that treatment with SLN-DTX was able to prevent tumor. IL-6 serum levels, ki-67 and BCL-2 expression were analyzed and showed a remarkably strong reduction when used in a combined treatment.
Conclusions: These results indicate that DTX-loaded SLNs may be a promising carrier to treat breast cancer and in metastasis prevention.
Keywords: 4T1; BCL-2; Cellular uptake; IL-6; Ki-67 and antitumor effect; NIH-3T3.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures









References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

